NCATS Launches Strategic Planning Process
The strategic planning process began in early 2015 with an extensive internal consultation phase among NCATS staff.
Message from the NCATS Director
Several thousand diseases affect humans, of which only about 500 have approved treatments. Thanks to our growing understanding of human biology, along with increased availability of innovative technologies, there is now an unprecedented opportunity to translate scientific discoveries more efficiently into new, more effective and safer health interventions. To empower the realization of this opportunity, NCATS develops innovations that reduce, remove or bypass costly and time-consuming bottlenecks in the translational science process, to speed the development and delivery of new drugs, diagnostics, medical devices and behavioral interventions to patients. Read the full director’s statement.

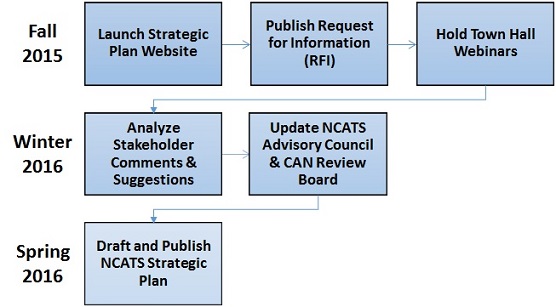
Strategic Planning Process and Timeline
NCATS is in the process of developing its first strategic plan to identify critical challenges, compelling opportunities, and emerging and unmet needs; develop and set scientific and operational goals and research priorities; and engage a diverse and broad community of stakeholders. The end result will be a living — that is, a purposely evolving — document charting an actionable path to effectively advance the NCATS mission.
The strategic planning process began in early 2015 with an extensive internal consultation phase among NCATS staff. On Oct. 8, 2015, NCATS launched the external engagement phase of the process to solicit feedback from the Center’s many stakeholders to help identify the highest priority challenges, opportunities and research needs in translational science. A public request for information (RFI) and a series of “town hall”-style webinars are the principal vehicles to collect this feedback.
NCATS encourages broad and diverse audiences to provide ideas for how the Center should work to most effectively advance translational science. This input will be critical in formulating and drafting the NCATS strategic plan.
Request for Information
NCATS published an RFI on Oct. 8, 2015, to collect stakeholder input on the scientific and operational opportunities, challenges and research needs in translational science. This information will help set the Center’s strategic priorities and inform the development of a five-year strategic plan. The deadline for comments is Jan. 8, 2016.
Town Hall Webinars
In addition to the RFI, stakeholders and interested parties can provide input via a series of “town hall”-style webinars to be hosted on the following dates and times:
- Monday, Oct. 26, 2015, 2:00-3:30 p.m. ET: Register Now
- Wednesday, Nov. 4, 2015, noon-1:30 p.m. ET: Register Now
- Tuesday, Nov. 10, 2015, 1:00-2:30 p.m. ET: Register Now
Individuals who register will receive a confirmation e-mail with information about how to participate online or by telephone. During the webinars, participants will be able to ask questions on issues related to the development of the strategic plan.
More Information
Send additional comments or questions to NCATSstrategicplan@mail.nih.gov.