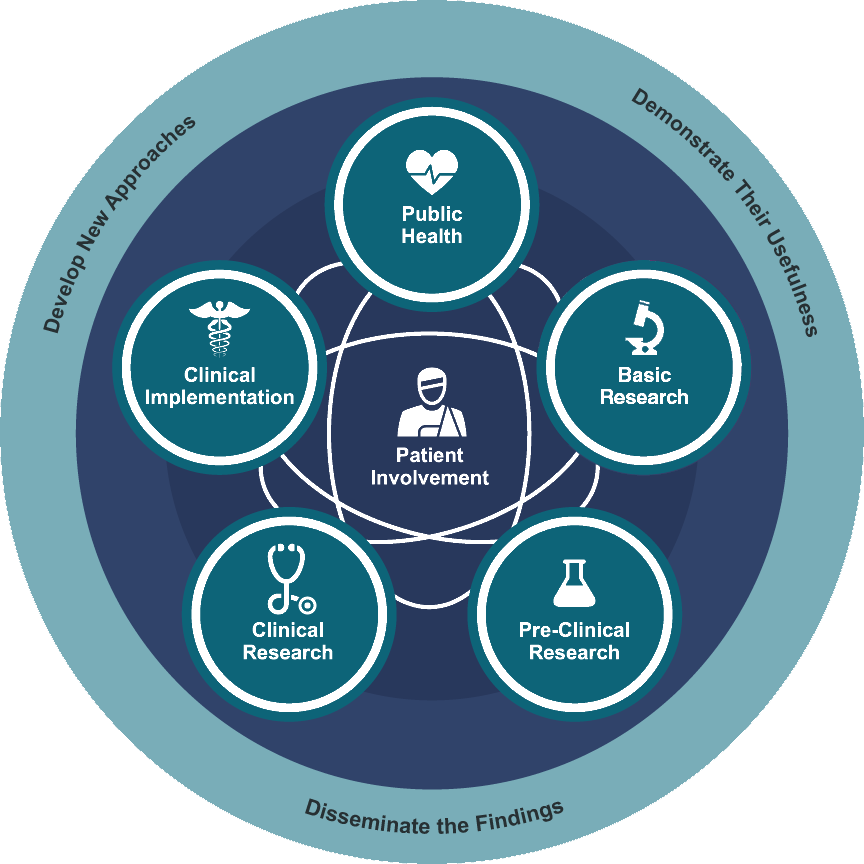
The translational science spectrum represents each stage of research along the path from the biological basis of health and disease to interventions that improve the health of individuals and the public. Researchers at this CTSA hub and across the globe face common barriers in translational research that can delay the development of new interventions for patients in need. Across the spectrum of translational science, the SC CTSI is focused on research involving people. This includes clinical trials, community partnered research, implementation science, and dissemination.
Translation describes the process of turning observations in the laboratory, clinic and community into interventions that improve the health of individuals and the public — from diagnostics and therapeutics to medical procedures and behavioral changes. Translational science is the field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process.
Basic Research
Basic research involves scientific exploration that can reveal fundamental mechanisms of biology, disease or behavior. Every stage of the translational research spectrum builds upon and informs basic research. NCATS scientists typically do not conduct basic research; however, insights gained from the Center’s studies along the translational spectrum can inform basic research.
Pre-Clinical Research
Pre-clinical research connects the basic science of disease with human medicine. During this stage, scientists develop model interventions to further understand the basis of a disease or disorder and find ways to treat it. Testing is carried out using cell or animal models of disease; samples of human or animal tissues; or computer-assisted simulations of drug, device or diagnostic interactions within living systems.
Clinical Research
Clinical research includes studies to better understand a disease in humans and relate this knowledge to findings in cell or animal models; testing and refinement of new technologies in people; testing of interventions for safety and effectiveness in those with or without disease; behavioral and observational studies; and outcomes and health services research. The goal of many clinical trials is to obtain data to support regulatory approval for an intervention.
Clinical Implementation
The clinical implementation stage of translation involves the adoption of interventions that have been demonstrated to be useful in a research environment into routine clinical care for the general population. This stage also includes implementation research to evaluate the results of clinical trials and to identify new clinical questions and gaps in care.
Public Health
In this stage of translation, researchers study health outcomes at the population level to determine the effects of diseases and efforts to prevent, diagnose and treat them. Findings help guide scientists working to assess the effects of current interventions and to develop new ones.