Development and Manufacture of Drug Substances (FDA Guidance Document)
CGMP – Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs (Code of Federal Regulations)
CGMP – Current Good Manufacturing Practice for Finished Pharmaceuticals (Code of Federal Regulations)
Q13 Continuous Manufacturing of Drug Substances and Drug Products (FDA Guidance Document)
eDRLS: Electronic Drug Registration and Listing System (FDA) - Register drug establishment and list products
Drug Establishments Current Registration Site (FDA) - Look up registered establishments

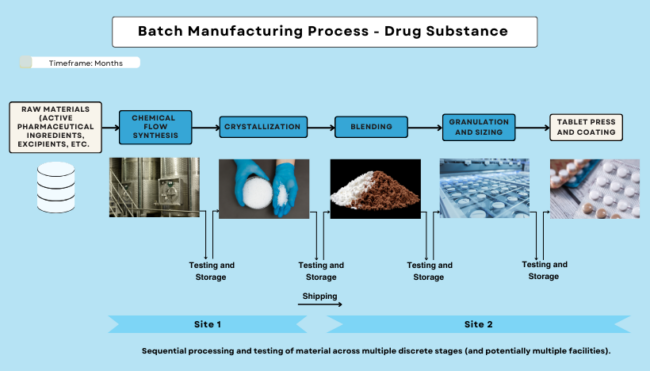
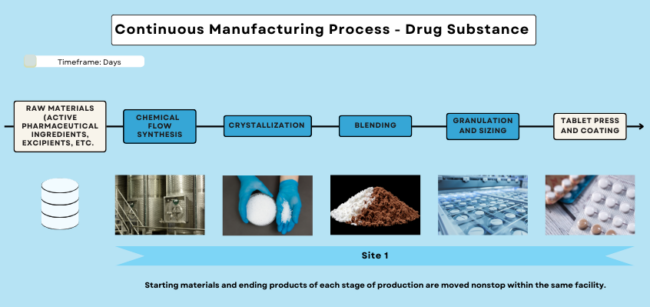
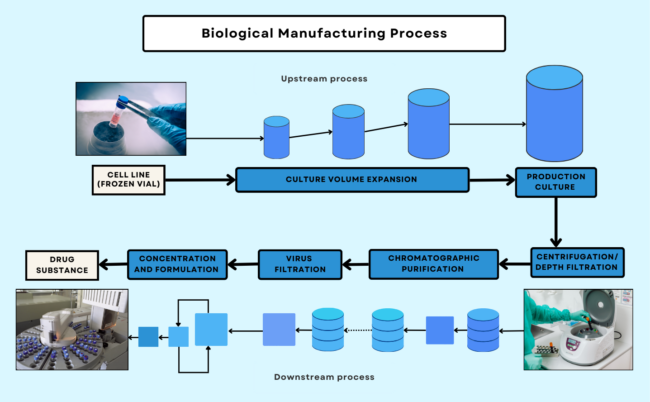
Manufacturing broadly refers to the general production of a medical product. While the process varies among pharmaceuticals/drugs, medical devices, and biologics/biosimilars, there are shared manufacturing principles and practices followed by manufacturers across all medical product types. In Figures 1, 2, and 3, you can see batch, continuous, and biological manufacturing processes, respectively. For information on devices, visit the Manufacturing Devices section.
Manufacturers must comply with Current Good Manufacturing Practices (CGMP), which refers to the regulations set forth by the FDA to ensure the “proper design, monitoring, and control of manufacturing processes and facilities” of medical products. These regulations outline production standards and include the minimum requirements for the methods, facilities, and controls used in the manufacturing, processing, and packaging of medical products. The FDA enforces CGMP compliance through surveillance inspections of manufacturing facilities to evaluate their compliance to CGMP as well as application-based inspections as part of a marketing application filed with the FDA. Note that it is more challenging to test the safety and potency of a biologics’ final products. Unlike traditional drug products composed of pure chemical substances, biologics derive their starting material from living systems. Because of this, the FDA monitors the manufacturing of biologics from its early stages paying close attention to any changes to the process, equipment, or facility that can affect the product.
Additionally, the FDA requires registration of domestic and foreign companies (or establishments) that manufacture, repack, or re-label drug and biologic products, including vaccines, and devices marketed in the United States. These companies are also required to provide a thorough inventory of all their commercially distributed products.
 |
 |
 |
Resources
Click each title below to reveal the resources
Developing and Manufacturing Drugs Including Biologics (FDA)
CGMP – Biological Products: General (Code of Federal Regulations)Biological Product Deviation Report
Manufacturing and Testing of Monoclonal Antibody Products (FDA)
Tissue Establishment Registration (FDA) - Register tissue establishment and list products
HCTERS: Human Cell Tissue Establishment Registration (FDA) - Look up registered establishments
BER: Blood Establishment Registration and Product Listing (FDA) - Register blood establishment and list products
Go to Resources for this page