Classify Your Medical Device
Quality Systems (QS) Regulation/Medical Device Good Manufacturing Practices
CFR Title 21 Subpart 820 QS Regulation Sec 820.3 Definitions
Device Registration and Listing Register medical device establishment and list products
Device Labeling
GUDID: Global Unique Device Identification Database Reference catalogue for every device with a unique device identifier (UDI)
Mandatory Reporting Requirements: Manufacturers, Importers, Device User Facilities
ISO 14971 Risk management standards for medical devices
Medical Device Development Tool (MDDT)
Software as a Medical Device
Digital Health Center of Excellence
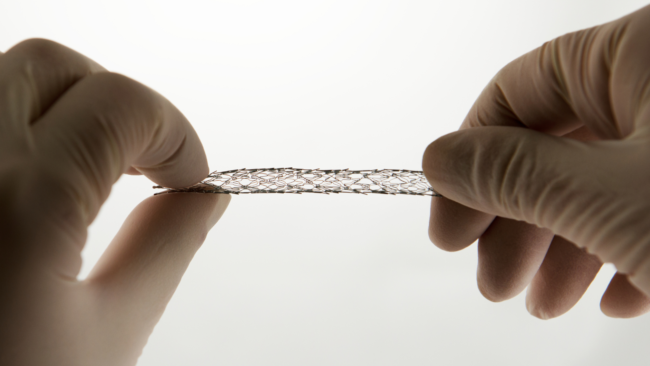
Manufacturing encompasses the comprehensive process of producing medical products, which can vary significantly for pharmaceuticals/drugs, biologics/biosimilars, and medical devices. For devices, manufacturers are required to establish and adhere to quality systems (QS) to ensure that their products consistently meet pertinent requirements and specifications as outlined in 21 CFR 820.
The Quality System (QS) regulation adopts a similar comprehensive approach as the original Current Good Manufacturing Practices (CGMP) regulation. It covers various aspects, including methods, facilities, controls, design, manufacturing, packaging, labeling, storage, installation, and servicing of medical devices for human use. The QS regulation applies to both finished device manufacturers and accessory manufacturers planning to commercially distribute medical devices. It also introduces preproduction design controls to align with global quality system requirements. Each manufacturer is responsible for establishing requirements for each device type or family to ensure their safety and effectiveness, and for creating methods and procedures that adhere to the quality system requirements. Even medical devices produced under an investigational device exemption (IDE) are not exempt from design control requirements under 21 CFR 820.30 of the QS regulation. The Office of Compliance (OC) within the Center for Devices and Radiological Health (CDRH) and the Office of Product and Quality (OPEQ) will simultaneously review QS information submitted in premarket submissions. Similarly, the Center of Biologics Evaluation and Research (CBER) regulates medical devices related to licensed blood and cellular products.
Additionally, the FDA requires registration of domestic and foreign companies (or establishments) that produce and distribute medical devices intended for use in the United States. These companies must also provide a list of all their commercially distributed devices and the activities performed on those devices at their establishment.
Resources
Click the title below to reveal the resources
Go to Resources for this page