An informational session about this funding opportunity was held on September 8, 2022. You may view the recording here.
Introduction
- Multidisciplinary Pilot Grants
- Award
- Priority
- Eligibility
- Multiple Awards
- Resubmissions
- Previous Awardees
- Letter of Intent (LOI) Instructions
Application
Introduction
Multidisciplinary Pilot Grants
Overview: This year, the Southern California Clinical and Translational Science Institute (SC CTSI) will concurrently offer two types of pilot grants: (a) Standard Pilot Grants of up to $50,000 each and (b) Multidisciplinary Pilot Grants of up to $125,000 each, both focused on clinical and community-engaged research. This announcement pertains to the Multidisciplinary Pilot Grant. Click HERE to view the Standard Pilot Grant announcement and information.
Purpose: These awards are intended to support newly formed, multidisciplinary teams conducting a new endeavor that is beyond the scope of one principal investigator or laboratory. This funding broadly supports the development of new Clinical and Community-based research projects in either/both of two areas: (a) collection of initial data in the areas of clinical, community or health systems research to support extramural grant applications and/ or (b) development and testing of new approaches and/or tools to increase the speed, efficiency, safety and/or quality of clinical, community, health outcomes and/or implementation research. Based on priorities of NIH for Clinical and Translational Research Awards, particular emphasis will be given to the latter category. Award funds will be distributed in two installments, with receipt of the second installment dependent upon meeting of bold interim metrics by 6 months. This mechanism is not intended to fund basic or preclinical research.
Virtual Information Session
Watch the info session about the pilot program that was held on August 31, 2023:
Research eligible for this call includes:
- Preliminary research conducted by a multidisciplinary team with at least two multi-PIs (MPI) that will lead to larger extramurally-funded human mechanistic studies, clinical trials, community trials, health outcomes research, or implementation research.
- Development of new approaches and/or tools to improve clinical and community-based research outcomes. Such proposals should clearly identify challenges or barriers and propose the development of a solution/process that can be tested within the pilot project.
NEW to the Scientific Review Process
Two stage submission process: All interested applicants must submit a letter of intent (LOI) by September 22, 2023, which will be peer reviewed by an Executive Committee. Up to 15 LOI applicants (combined, from standard and multidisciplinary pilot applications) will be invited to submit a full pilot proposal following the guidelines below, which is due November 13, 2023.
(Scroll down on this page to find the LOI Submit button)
Full proposals reviewed by a panel of scientists & community members: Review panels will include scientific reviewers with relevant project-specific expertise, as well as community members who have undergone comprehensive reviewer training. Both scientific and community reviewers will read, score, and participate in discussion of the applications. This purpose of this community-engaged review panel is to help meet the SC CTSI’s goals to integrate community perspectives in shaping our research agenda and to be able to communicate to the public about work funded at SC CTSI.
If you have any questions regarding this RFA, please contact rd@sc-ctsi.org.
Award
Applicants may request up to $125,000 for a 12-month period beginning April 1, 2024, composed of a 4:1 ratio of funds from SC CTSI to funds from the investigators’ home department(s), school(s) or Institute(s). For example, the maximum award would be $100,000 from SC CTSI funds and $25,000 from the home department(s), school(s) and/or institute(s) of the participating investigator(s). Applications must include a letter or letters of commitment specifying the amount and source(s) of the cost share. Applications from multiple departments can derive the cost share in any mix from the participating departments; it is up to the investigators to secure the matching funds to be made available in a USC account at the time of funding award. Award funds will be distributed in two installments, with receipt of the second installment dependent upon meeting of 6-month interim metrics.
Priority
Preference and highest priority will be given to projects that demonstrate:
- A multidisciplinary approach and engagement of multiple investigators across disciplines in a new research partnership.
- A clear path to sustaining extramural funding or to direct implementation of improvements in clinical and community-engaged research processes.
- The ability to move forward rapidly and to articulate and meet bold interim metrics by 6 months.
- Commitment of the PI to a career in research will be considered a plus. Proposals will be evaluated using an NIH-style peer review process.
Eligibility
Full-time faculty members at USC and CHLA as of the date of the LOI submission (September 22, 2023) are eligible to apply as PI. This mechanism requires multi-PIs, with each co-PI’s expertise contributing to the multidisciplinarity and breadth of the team. Each multi-PI must devote at least 30% of their professional effort to research. As confirmation of this, a letter from the department chair of each multi-PI must be provided confirming the specific time that will be made available for the PI to complete their research while also fulfilling any other teaching and/or clinical commitments.
- Postdocs, residents, fellows, students, and community members can be included as co-Is.
- International Faculty who are non-US citizens/permanent residents must have a USC appointment and currently hold a visa to allow them to remain in the US long enough to complete the proposed project.
Multiple Awards
Investigators may only submit one application in which they are listed as PI or multi-PI for either the Standard Pilot Award or the Multidisciplinary Pilot Award, not for both. However, a PI can be on more than one grant submission to either mechanism if he/she/they is listed as a co-investigator.
Resubmissions
Resubmissions of previous SC CTSI Pilot applications will not be accepted.
Previous Awardees
If the investigator(s) have previously received SC CTSI pilot funds, a summary of the progress made on the previous award including publications, grant submissions, funded grants, and other evidence of progress and impact must be included with the LOI. This summary can be up to 1 page.
Letter of Intent (LOI) Instructions
Interested applicants must provide three items:
- One-page LOI
The LOI should briefly describe: (i) the project goals/specific aims and its alignment with the goals articulated for the program above, (ii) the project team, including expertise and contributions of key personnel to the multidisciplinary project, (iii) any team science activities planned to improve team communications and function across disciplines, and (iv) how the project will lead to larger extramurally funded research. The LOI must be written in 11-point Arial font with 0.5-inch margins.
- NIH-formatted biosketches from all proposed key personnel
(Link to NIH Biosketch guidelines template: https://grants.nih.gov/grants/forms/biosketch.htm)
- A brief personal statement
Applicants will also provide a brief (up to ½ page) personal statement that describes the PIs expertise, research experience including experience with community-engaged research, publication and grant record, and career goals for conducting independent research.
LOIs will be reviewed by an Executive Review panel for significance, innovation and fit to the mechanism. Up to 15 LOIs (combined, from standard and multidisciplinary pilot submissions) will be selected to advance to full submission. Note: Previous SC CTSI pilot fund awardees must include a summary of their progress on the previous grant(s), as described above (this is in addition to the one-page LOI).
To be considered for funding, LOIs must be submitted by September 22, 2023, at 11:59 PM (PST).
Letter of Intent example #1 Letter of Intent example #2
Application
Full Application Content & Instructions
If selected to submit a full proposal, applicants will be required to connect with specific SC CTSI core groups as specified by the Executive Review panel to receive advising during the development of their proposals and prior to submission. Recommendations for the specific core groups aligned with the goals of the proposal will be provided to successful LOI applicants in the first week of October, along with the invitation to submit a full proposal. Applicants will report on the SC CTSI groups they engaged with and the impact on their proposals in a special section of the application portal. A complete application will include the following components arranged in the specified order. The applicant is responsible for the readability of the entire application.
Complete applications must be submitted by November 13, 2023, at 11:59 PM (Pacific). Submission instructions will be provided upon LOI Selection.
Format
- Arial font, 11pt
- Margins: minimum 0.5 inch
- Figure and Table legends may use smaller font, but no font smaller than 9pt is allowed to ensure readability.
- Please use appropriate headings in preparing your proposal.
- No appendices
- The application portal contains multiple upload fields for separate components of the application. Please upload only PDFs to the correct upload fields.
The application should include the following:
- Cover Letter (See multi-PI qualifications and commitment to a research career)
- Abstract (max 300 words)
- Lay Summary (written or visual). To aid our community reviewers, prepare a two-page summary of Sections A-D from the Research Proposal; in this summary minimize jargon and promote readability at a nonscientific lay level. Alternatively, you may prepare a 5-minute video which explains your proposed project to a general (lay) audience. The SC CTSI will provide you with further information for the video. Please contact rd@sc-ctsi.org to arrange file transfer.
- Research Proposal (maximum 5 pages, including figures/tables, excluding literature cited)
- Overall Impact and Significance
- Discuss the significance of this project in terms of innovation and impact on human health.
- Explain the potential for high impact, importance of the problem, and critical barriers.
- Describe the next steps in the research agenda if the proposed aims are achieved.
- Innovation
- Describe any novel theoretical concepts, approaches, or methodologies, as well as any novel instrumentation or interventions to be developed or applied in a novel way; describe any advantages over existing methods, instrumentation, or interventions.
- Explain any novel refinements, improvements, or applications of theoretical concepts, approaches, methods, instrumentation, or interventions.
- Specific Aims
- State the specific aims to address the research question.
- Approach
- Describe the overall strategy, methods, and analyses to be used to accomplish the specific aims of the project. Specifically describe how the data will be collected, analyzed, and interpreted. Describe resource sharing plans if appropriate.
- Discuss potential problems, alternative strategies, and milestones for success in achieving the aims.
- Describe your specific plan for how project findings can be implemented more broadly.
- If the project is in the early stages of development, describe any strategies to establish feasibility. Address the management of any high-risk aspects of the proposed work.
- Describe how the project will solidify new multi-disciplinary research collaborations.
- Describe plans for disseminating and/or commercializing the findings. Awardees will be invited to present their study results at a Keck School of Medicine and SC CTSI co-sponsored event.
- Overall Impact and Significance
- Next Steps (max 1 page)
i. Describe plans for implementing the project finding more broadly, including disseminating and/or commercializing the findings. NOTE: Awardees will be invited to present their study results at a Keck School of Medicine and SC CTSI co-sponsored event.
ii. Describe your specific plan of obtaining extramural funding. This should include what this project will provide with regard to the type of grant that will be targeted. Applicants are encouraged to name specific funding mechanisms if they have been identified - Team Building Plan & Objectives (max 2 pages)
- Team Composition – highlight diversity of the team including as relevant the incorporation of early stage and established researchers, multidisciplinarity, sectoral diversity (scientist, patient, community), as well as diversity of experience.
- Team Roles – provide a clear description of the unique contributions of team members, any existing collaborative relationships between the team members, and how the team will be organized (e.g., roles, tasks, leadership).
- Team Building Activities Proposed
- A timeline or project plan with defined milestones. Bold and well-defined milestones to be met at 6 months for receipt of the second installment of funding should be clearly articulated; propose only what can be accomplished within ONE YEAR (max 1 page)
- Resources and Environment (max ½ page) Document access to any resources and environment that will be required for successful completion of the project.
- Literature Cited (not included in page limit)
- Budget ($125,000 maximum)
- Applicants must submit two budget forms for (1) the first six months and (2) the second six months of funding. Initial awards will be made for the first six months of funding. Remaining funding will be contingent upon meeting bold and clearly-articulated milestones that must be described in the timeline.
- Multi-PI and co-investigator salary support is not allowed.
- Use the Required PHS 398 Budget Form, Page 4 or download the Budget Form HERE. Award expenditures must comply with the NIH Grants Policy Statement and the Uniform Guidance OMB A-81. Indirect costs are not allowed, including sub-contracts funded by SC CTSI pilot awards. Sub-contracted institutions are required to waive indirect costs.
- Fringe benefits are at USC Rates and NOT federal
- Budget Allowable and Not Allowable Items
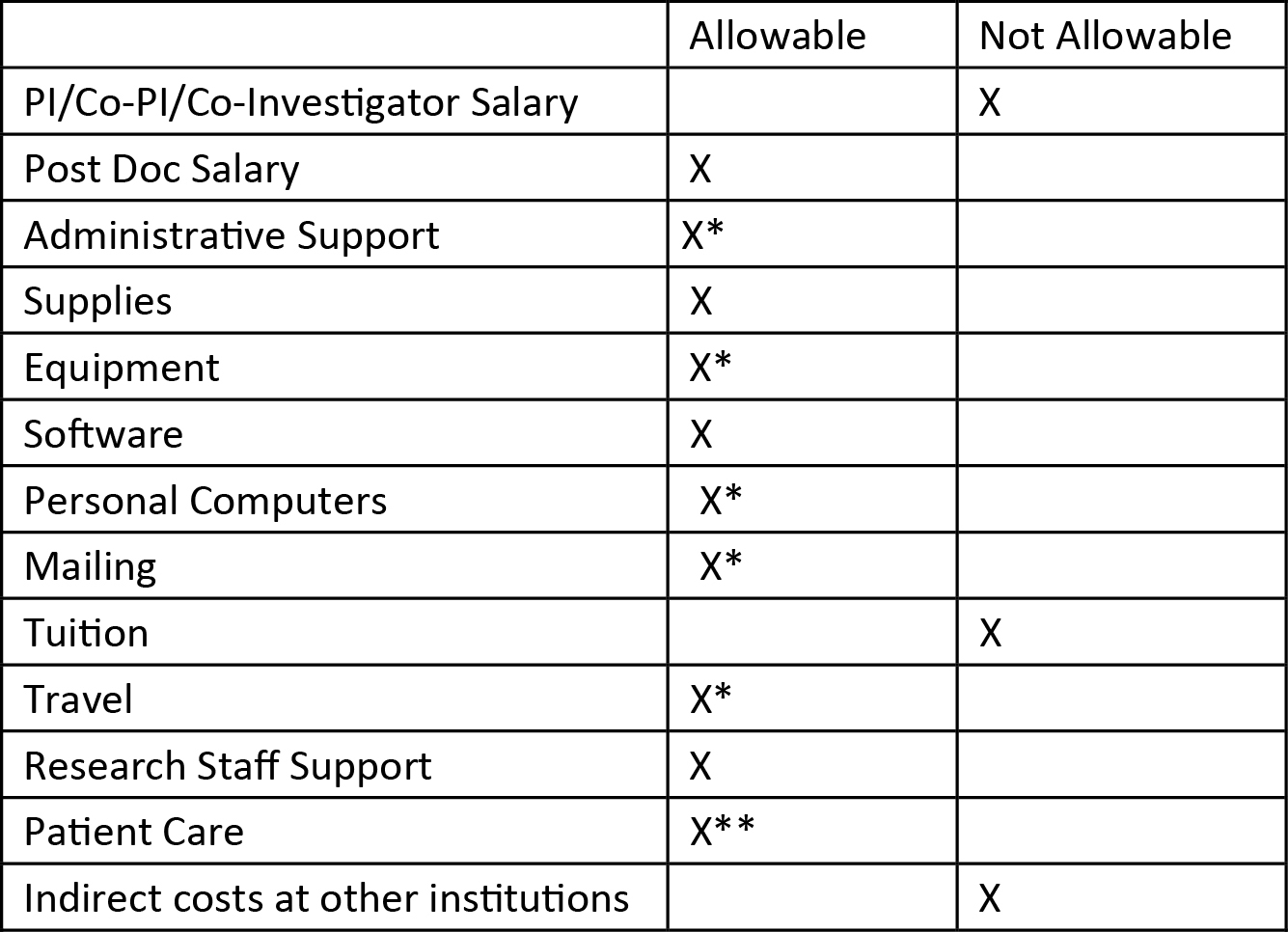
*These items are not favored by the grant mechanism but will be considered if specifically justified in the budget justification.
**Standard care costs are not allowable, but costs related to research procedures on study participants are if justified.
- Budget Justification Justify all costs and provide detailed information for all requested items.
- Regulatory Approvals NIH requires that all CTSA-funded projects involving human subjects obtain approval from the local IRB and from NCATS. To facilitate this process, awardees of projects involving human subjects are asked to submit local IRB proposal within two weeks of award notification. No funds will be distributed until the local IRB application has been submitted and no funds will be released for direct human subjects components of a project until local IRB and NCATS approval has been granted.
- NIH Biographical sketches of multi-Principal Investigator and/or Co-Investigators (5-page max for each individual) The NIH biographical sketch is required for all investigators. Specific roles of each multi-PI and co-investigator must be defined. (Please use latest NIH format found at https://grants.nih.gov/grants/how-to-apply-application-guide/forms-g/general/g.240-r&r-seniorkey-person-profile-(expanded)-form.htm#Instructions)
- Letter(s) of Support Applicants must include a letter of commitment from each multi-PI applicant’s home department Chair confirming that the applicant has at least 30% effort commitment to research (not just for this project, but for research overall) for at least 18 months after the proposed award date. Other letters confirming access to crucial resources including necessary space, release time and environment may be included here.
Timeline for Fall 2023 Cycle
- RFA Released: August 3, 2023
- LOI Submission Deadline: September 22, 2023
- Notification of LOI applicants invited to submit Full Proposals: October 6, 2023
- Full Proposal Deadline: November 13, 2023
- Review Period (November 14- December 18, 2023)
- Final Selection Made: January 2024
- Submission of regulatory approvals to NIH: January – April 2024
- Funding Period: April 1, 2024 – March 31, 2025
Award Conditions
- Six Month Review Funded projects will initially receive funding for the first six months of the proposed project. Funded projects will be expected to submit a progress report near the end of the first six months of the award term. Progress reports should summarize progress toward scientific goals and pre-specified project milestones. The second six months of funding will be released when it is determined that sufficient progress has been made on project milestones. SC CTSI Staff will offer support to trouble shoot barriers to support the success of the funded projects.
- Regulatory Approvals NIH requires that all CTSA-funded projects involving human subjects obtain approval from the local IRB and from NCATS. To facilitate this process, awardees of projects involving human subjects are asked to submit local IRB proposal within two weeks of award notification. No funds will be distributed until the local IRB application has been submitted and no funds will be released for direct human subjects components of a project until local IRB and NCATS approval has been granted. Human Subjects (IRB) and other regulatory approvals should indicate that your research is/will be supported by SC CTSI Grant UL1TR001855.
- Pilot Launch Support SC CTSI has developed a new Pilot Launch Program intended to position awardees to successfully execute their studies. This will include training on Team Science and best practices, as well as participation in the SC CTSI’s new Quality by Design (QbD) program. The QbD framework defines quality as the absence of errors that impact the safety of trial participants or the credibility of the trial results. Potential errors are identified at the study design stage with input from a wide range of experts and stakeholders. Risks are closely monitored and addressed throughout the conduct of the study. The project team for each awarded project is required to participate in the Pilot Launch Support program, which will be initiated prior to funding in Spring 2024.
Lay Summary Resources & Guidance
Click HERE for video instructions.
Watch the following videos on how to record from your computer:
• HP Computers:
• Macbook Pro:
Lay Summaries Tips from Elsevier:
https://www.elsevier.com/connect/authors-update/in-a-nutshell-how-to-write-a-lay-summary
- Predict and cover the “so what?” factor – justify your research.
- Give some background and context to the research. What prompted you to do it?
- Follow a logical order. This may not always coincide with a temporal order.
- Explain the impact of the work – what is going to change (especially in relation to wider society)?
- Use succinct, short sentences – and write and/or speak in plain English. Imagine you’re talking to an undergraduate who’s just stepped into your introductory class. Or, better still, pretend you’re trying to explain your article to a distant family member who works in retail/fashion/hospitality.
- Avoid jargon unless absolutely necessary and explain it if you do have to keep it in.
- Use first person and active voice (“we agreed” rather than “it was agreed”).
- Use positives not negative sentences: “You will have repeat appointments at least once a week”, rather than “The usual practice is not to schedule repeat appointments more frequently than once a week”
- Images are very important – try to include one if you can.
If you have any questions regarding this RFA, please contact rd@sc-ctsi.org.
Contact information
Research Development at SC CTSI
(323) 442‑1087
Documents
SC CTSI Fall 2023 Multidisciplinary Pilot Grant Program RFA
Questions?
Try the Multidisciplinary Pilot Award FAQ page.
Awards and Other Funding Opportunities
Team Building Funding Opportunity
Mentored Career Development Opportunity
Other Services