Clinical Trials Unit: Conduct A Study
SC CTSI provides modern clinical infrastructure and trained research staff to help researchers conduct clinical studies.
Service description: The SC CTSI Clinical Trials Units provide an efficient clinical infrastructure, services, and resources for the conduct of early-phase (drug development) and complex mechanistic clinical trials. The units support outpatient and inpatient research and ensure the compliance with local and federal regulations.
Locations: Keck Medical Center of USC (USC CTU) | Children’s Hospital of Los Angeles (CHLA CTU)
Study Participant Room at the SC CTSI Clinical Trials Unit. View photo gallery
Trained clinical research staff, including research nurses, study coordinators, phlebotomists, radiology technician, assists with services such as:
- Nursing Services (Inpatient/Outpatient)
- Sample procurement
- Specimen processing
- Dexa scan, EKG, infusion equipment
- Handling laboratory
- Bionutrition evaluation
- Neurologic evaluation
- Metabolic testing
- Assay development and performance
- Secure and limited access investigational product storage area
- Parking for research participants
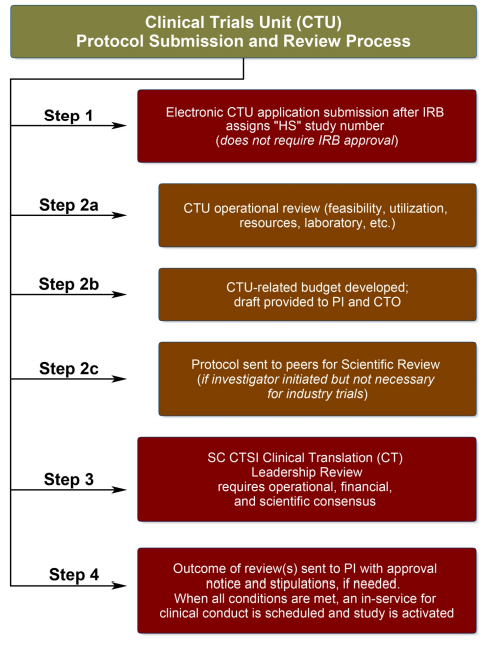
Learn more & Request the service The complete process takes approximately 45 days, from day of submission to approval/dissent notice.
Need help with developing a study protocol, budget, or IRB approval? Request a consultation
View the Guide to Clinical Research at USC for more information