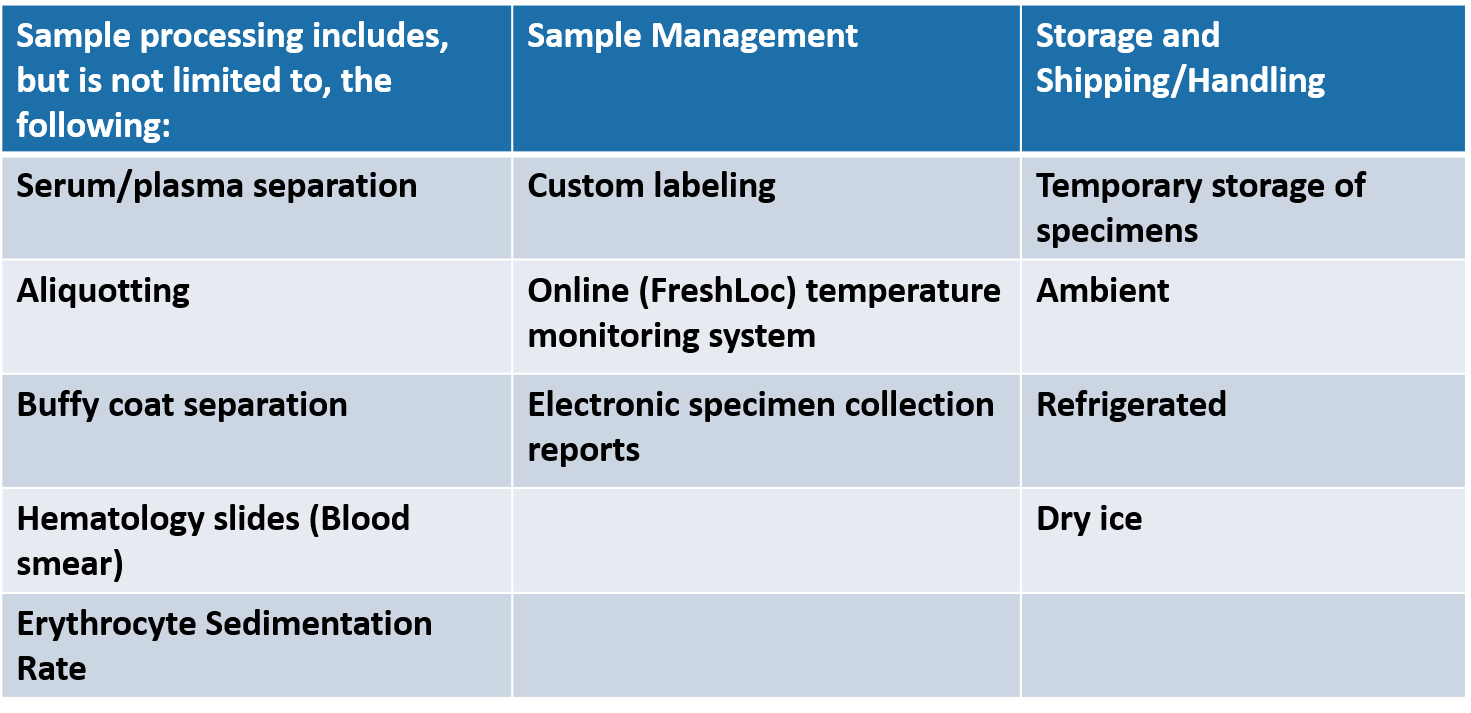
The CTU Specimen Processing Center serves as a one-stop unit for processing, storing, and shipping research samples collected from research patients.
In addition, the Center is able to perform specialized processing for studies in which the assays are performed in the investigator’s lab, research core lab, or sponsor’s central lab.
Specimen Processing
A one-stop unit for processing, storing, and shipping research samples collected from research patients
Service description
CTU Specimen Processing Center At-a-Glance
Clinical Trials Unit (CTU)
Provides a broad range of services to assist in the initiation and conduct of human services
Tooltip: Clinical Trial Management System (CTMS)
USC’s Clinical Trial Management System, OnCore, is a jointly sponsored, web-based software system for managing clinical trials.