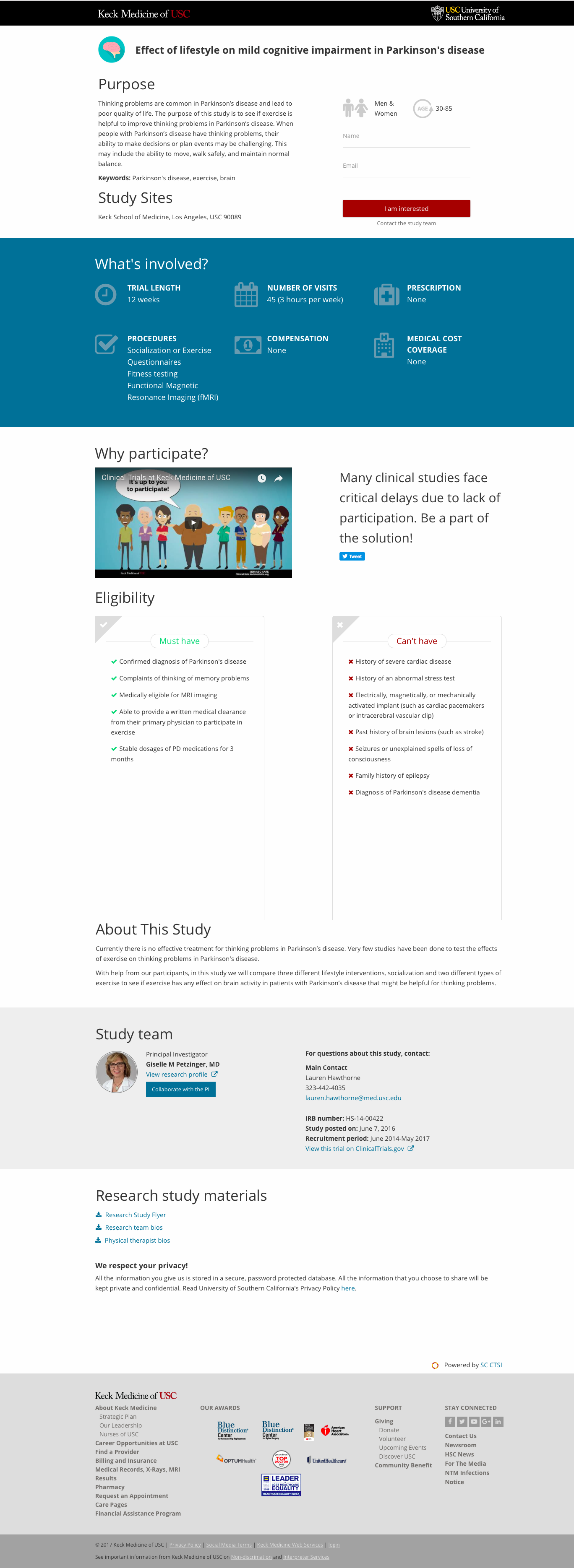
The USC Clinical Studies Directory is a free service and makes it easy for investigators to create Web pages that describe their research studies and facilitate the contact with the study team in an automated fashion. If your study is on ClinicalTrials.gov the system automatically creates a page but you can also create more user-friendly pages for any study. However, you can create more enhanced pages such as the one shown below.
USC Clinical Studies Directory
Helping USC researchers to connect with prospective clinical study participants
Tool description
Access
This tool is open to any USC researcher conducting studies that involve human participants.
Creating customized study pages
- In order to create landing pages for listing in the Clinical Studies Directory of USC, you will first need to sign up for an account here: https://clinicaltrials.sc-ctsi.org/research_team/sign_up
- You will receive an email asking you to verify your account. After verifying your account by clicking on the link in the email, you will be taken to the login page where you can sign in and start creating landing pages for your clinical trials.
- You can return to your account at any time by visiting this page: https://clinicaltrials.sc-ctsi.org/users/sign_in?locale=en
- When you log in to your account, you will be taken to your dashboard. Here you can create a new clinical study landing page as well as continue editing landing pages that you’ve previously created.
- When you initially create a landing page, you will be asked to enter a title and the name of the PI. The PI will be sent an email asking them to verify that you are authorized to create a landing page for their study. Please note that you will be unable to edit the page until it has been verified.
- Once a landing page has been verified by the PI, you can continue to edit the landing page as often as you need until it is ready for approval by the IRB.
- Click on the edit icon on the dashboard to continue editing a landing page you’ve previously created.
- We’ve included tips to help you create an engaging landing page. Click the tips while editing a study page to get instructions and suggestions for writing successfully to a lay audience.
While creating your study page, you might find that you need to share a read-only version of this page with other members of your study team or your PI in order to get feedback. You can click on the title of your study on the dashboard in order to view a read-only version of your page. You can share the URL of this page with anyone you need to.
Once you are satisfied with your study page, click on “Save and notify admin for review.” A member of our team will look over your page and provide feedback. Once they have given their approval, you can go ahead and submit your landing page for approval by the IRB. Submit an amendment to the IRB containing a link to the study page as well as screenshots of the page itself. This process usually takes 1-2 weeks to obtain IRB approval.
- Landing pages that do not have proper IRB approval cannot be made publicly available. Pages that contain the exact same content as the ClinicalTrials.Gov page do not need IRB approval. If you make any modifications in language or content the page, you will need to submit to IRB for approval.
- Please submit a Word document to the IRB with all the content in the landing page in your amendment. The IRB might require some changes to the landing page before it can be made public.
- Once the IRB approves the landing page, upload the approval letter or screenshot of approval letter to crs@sc-ctsi.org.
- The IRB approval letter will be reviewed by CTSI administrators and if proper approval is provided, the page will be made publicly accessible within 24 hours. You will be able to view the link to your published page on your dashboard.
If you have any questions or encounter any difficulties, please email dic@sc-ctsi.org
Example of customized study page
Request this Service
Click HereGet Study Participant Recruitment Support
Obtain customized recruitment and retention strategies that fit your study’s needs, timeline, and budget.