Does Someone in Your Family Have a Serious Health Issue? Consider Participating in Clinical Health Research. Here's How.
USC's Clinical Studies Directory & Research Volunteer Registry make it easy to take part in research studies that might help you or a family member treat a health concern. You'd also be helping to advance medical science.
We all rely on clinical researchers and other scientists to develop new medicines and treatments for the health issues we and our families face. But the researchers also rely on you—members of the public. Without your generous help in the form of participation in clinical health studies, scientists have no way to develop and test important new drugs and other treatments that have the potential to improve health and save lives.
Maybe you or someone in your family has a serious health condition, such as diabetes or cancer, and you are eager to help advance medical science as well as potentially benefit from the newest therapies. Researchers need participants of all ages, races, genders and backgrounds to join clinical studies. So how can you participate?
If you live in Southern California, USC's online Clinical Studies Directory and Research Volunteer Registryis the place to search for the right studies, connect with the researchers themselves, and potentially join a clinical study.
These easy-to-use websites are run by Keck Medicine of USC, which is one of California's most important centers of health research, and another organization based at USC, the Southern California Clinical and Translational Science Institute (SC CTSI). The SC CTSI is part of the U.S. government's National Institutes of Health, and works in many ways to advance medical research. It also specializes in engaging the community in health research and improving the health of all people in the Los Angeles and Southern California community.
Participating in a clinical trial may require an investment in time and trouble, but it can be an excellent way to gain access to the field's top health and science specialists and to the latest medicines or therapies. In addition, the value to science, health, and the larger community is immeasurable.
"Clinical research is a partnership between the scientists in the laboratories and people from every community," said Katja Reuter, PhD, Director of Digital Innovation and Community at the SC CTSI. "What we want people to know is that their participation is vitally important to health science and research. We cannot do it without the community's help, including their knowledge and input."
But is it safe to take part in a research study? "Clinical health research is tightly governed by a large body of laws, regulations, and procedures designed to make sure that study participants are not harmed," said Reuter. "Ensuring the health and safety of study participants from our community is built into the design of all research studies."
Members of the public interested in participating in clinical research can use the Clinical Studies Directory and Research Volunteer Registry to search for current health studies and to make themselves available for participation in a study.
Here's how it works and how you can take part.
Image 1. Home page
This is what the home page for the Clinical Studies Directory and the Research Volunteer Registry looks like. It's where members of the public can learn how clinical research studies work and how scientists use clinical studies to develop much-needed new medicines and treatments. Perhaps most importantly, members of the community can learn why their participation is so crucial to scientific progress.
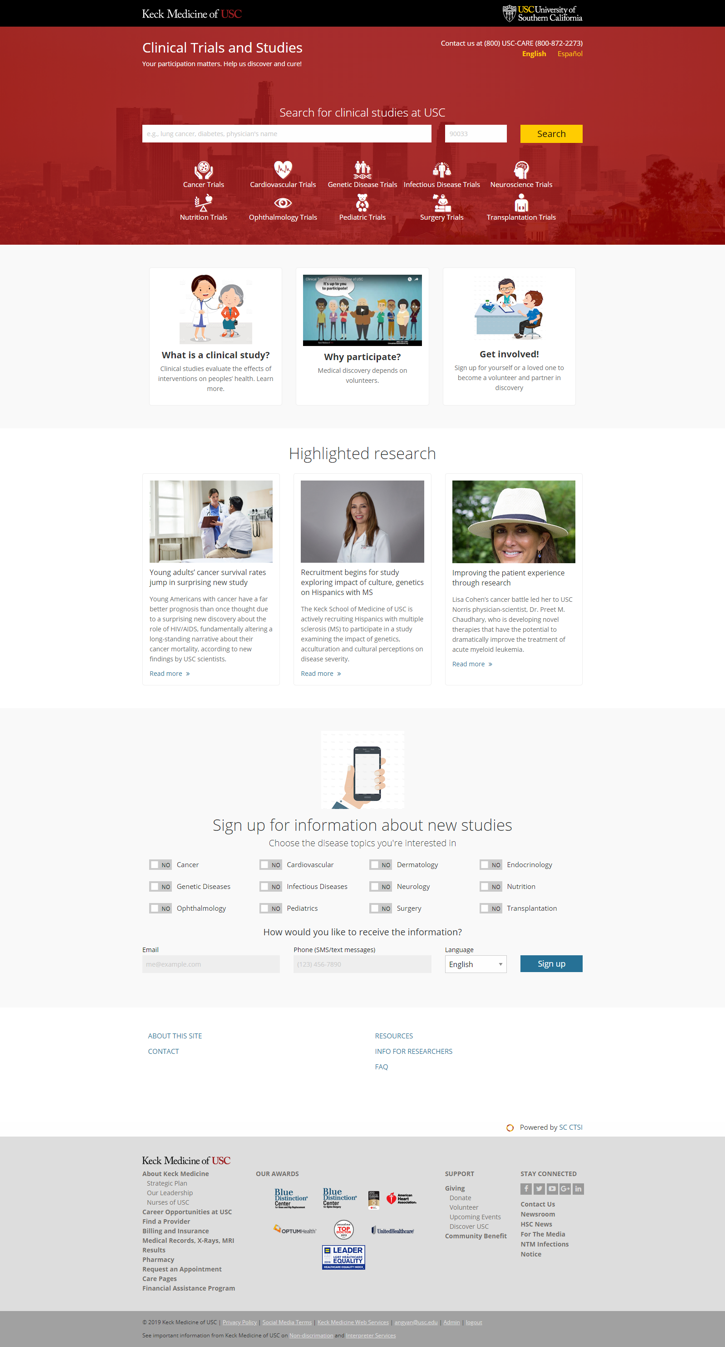
Image 2. Search Interface + Community Interest Areas
Improvements to the homepage Clinical Studies Directory and Research Volunteer Registry homepage give members of the public more options to search for the right clinical study to participate in. It is now possible to search the directory of ongoing studies based on keywords reflecting your health and treatment interests, including by disease, physician name, geographic location, or drug name.
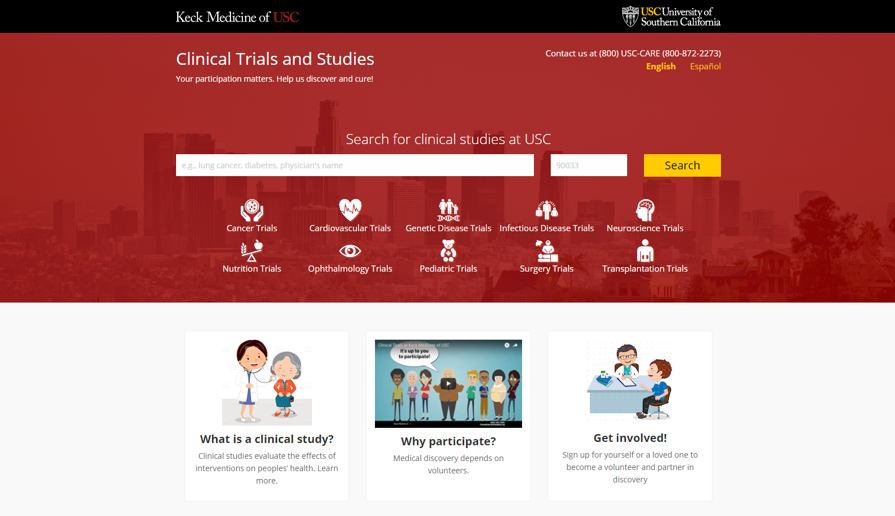
Image 3. Sign up to receive information about new studies
It is important that potential research study participants develop an understanding of the various studies in their health areas of interest, whether it is cancer, pediatrics, or endocrinology. The Clinical Studies Directory enables potential participants or family members to sign up to receive information about new clinical research studies. Following our recent upgrades, the system will now automatically email or text updates about new studies in the selected areas of interest.
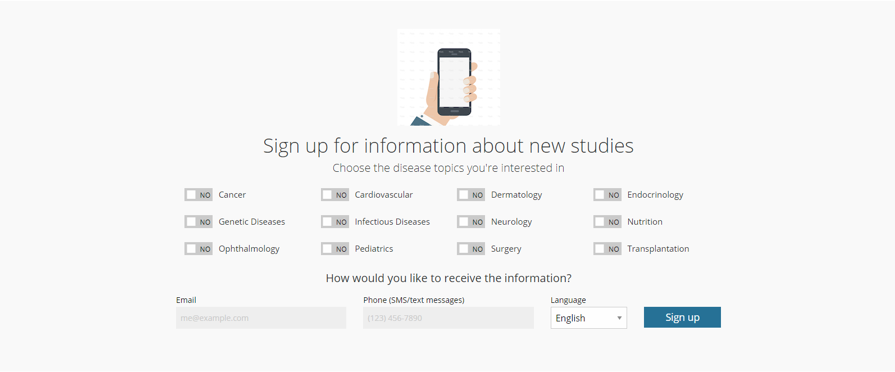
Image 4. Search for Ongoing Clinical Studies at USC
Potential participants can easily search a complete catalog of clinical studies in specific health conditions and diseases that are currently underway at USC. This is also a good way for community members to learn about how clinical research is conducted, and a great way to get up to speed on the latest treatments and drug candidates currently under study. Individual study pages provide more information about eligibility criteria, the principal investigators conducting the study, and how to contact the study team.
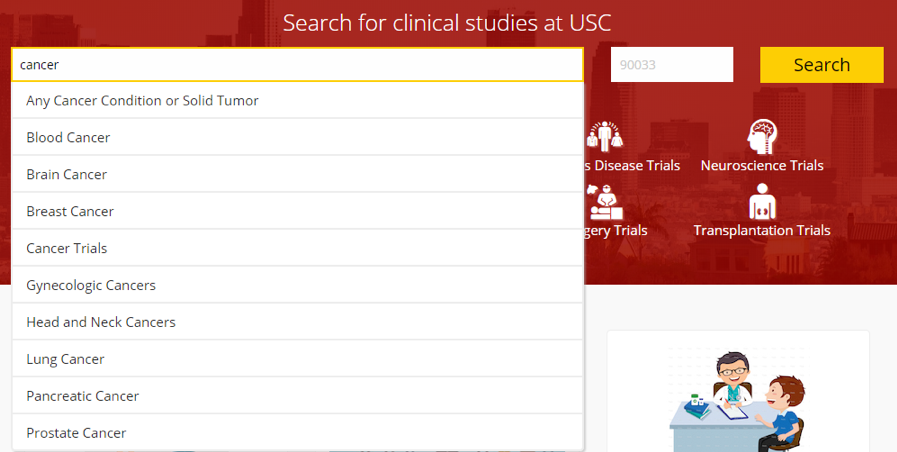
Image 5. Volunteer Registry
The Volunteer Registry is where members of the public can record relevant information about themselves, including health and medical records, and other basic information that can help researchers determine their eligibility for participation in a study.
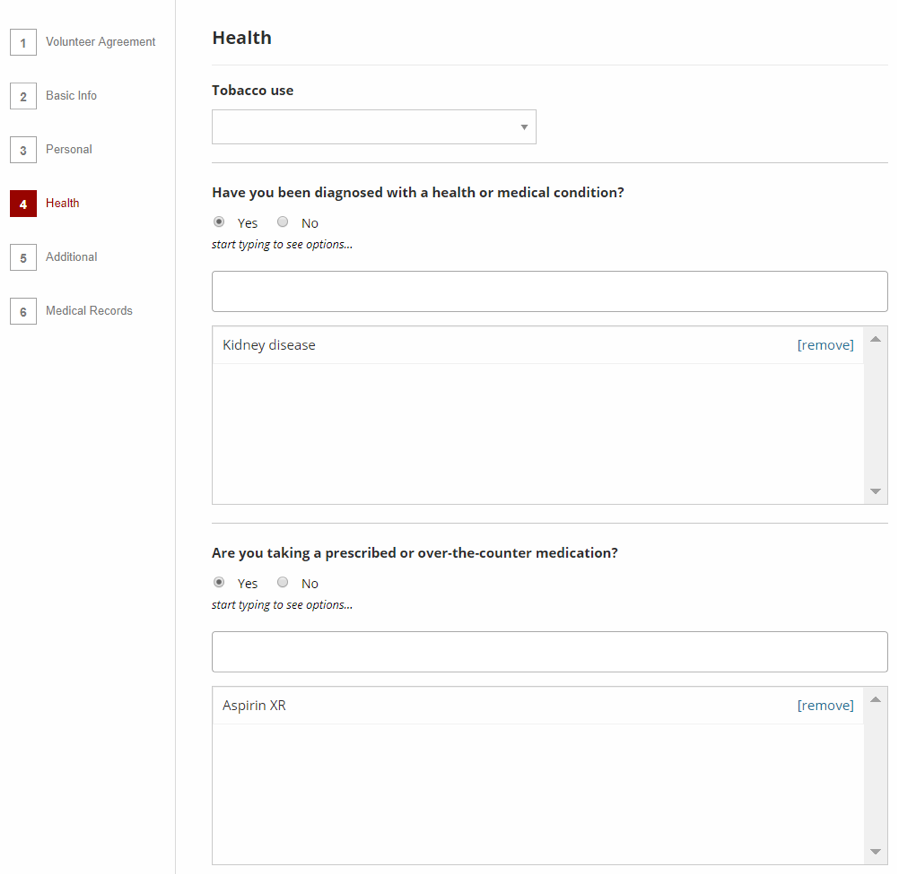
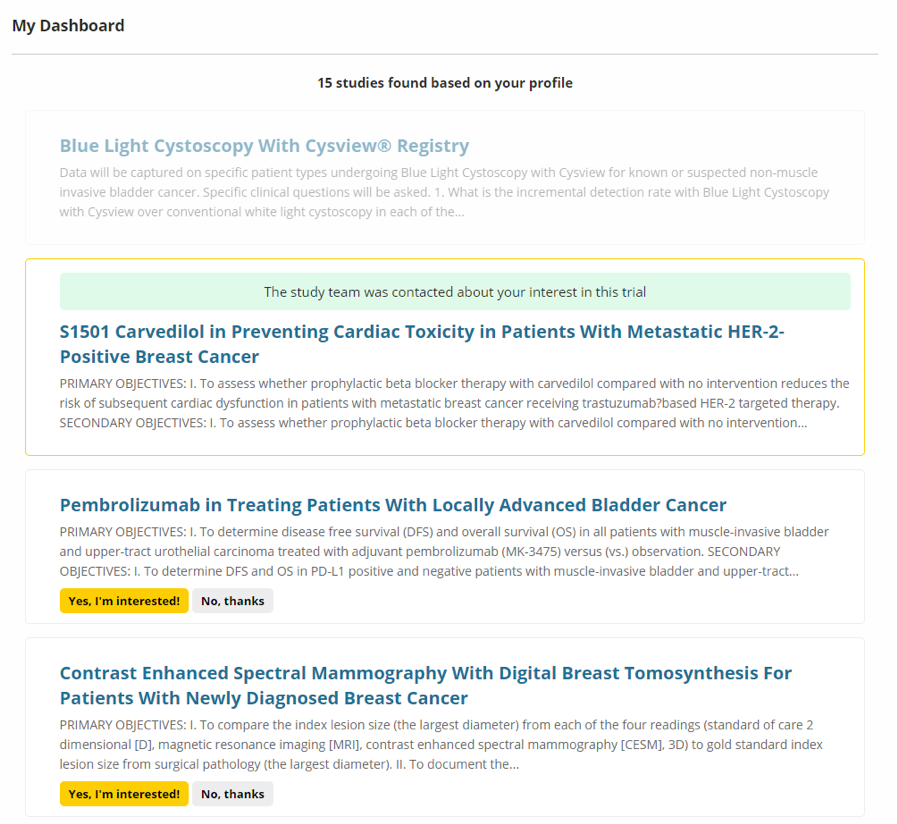
Image 6. My Dashboard
The Directory and Registry tools can match your personal profile with clinical studies. My Dashboard will provide a list of studies that match your health history and other personal criteria. You can read about the various research studies and decide if you might be interested in participating, and you can contact the research team. The decision to participate in any research study is always up to you.