ICMJE Journals Require Advanced Registration of Human Studies
Registration of clinical trials in a public trials registry is required at or before the time of first patient enrollment.
The International Community of Medical Journal Editors now requires, and recommends that all medical journal editors require, registration of clinical trials in a public trials registry at or before the time of first patient enrollment as a condition of consideration for publication. List of journals in ICMJE
ICMJE accepts registration in any registry that is a primary register of the WHO International Clinical Trials Registry Platform (ICTRP) or in ClinicalTrials.gov. Several USC researchers have been denied publication due to lack of registration.
The registration requirement applies to a broad definition of “clinical trials.” Health-researchers may be unaccustomed to applying this definition to their research. However, all human studies, as defined below, should register in clinicaltrials.gov to maximize the chance of publication:
“…any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes." Health-related interventions include any intervention used to modify a biomedical or health-related outcome (for example, drugs, surgical procedures, devices, behavioral treatments, dietary interventions, and process-of-care changes). Health outcomes include any biomedical or health-related measures obtained in patients or participants, including pharmacokinetic measures and adverse events.” Additionally, the FDA / NIH and CMS require study registration for all “applicable clinical trials” within ClinicalTrials.gov.
Online support with registration to clinicaltrials.gov | Or contact Jean Chan (jeanbcha@usc.edu, 323 442-2825)
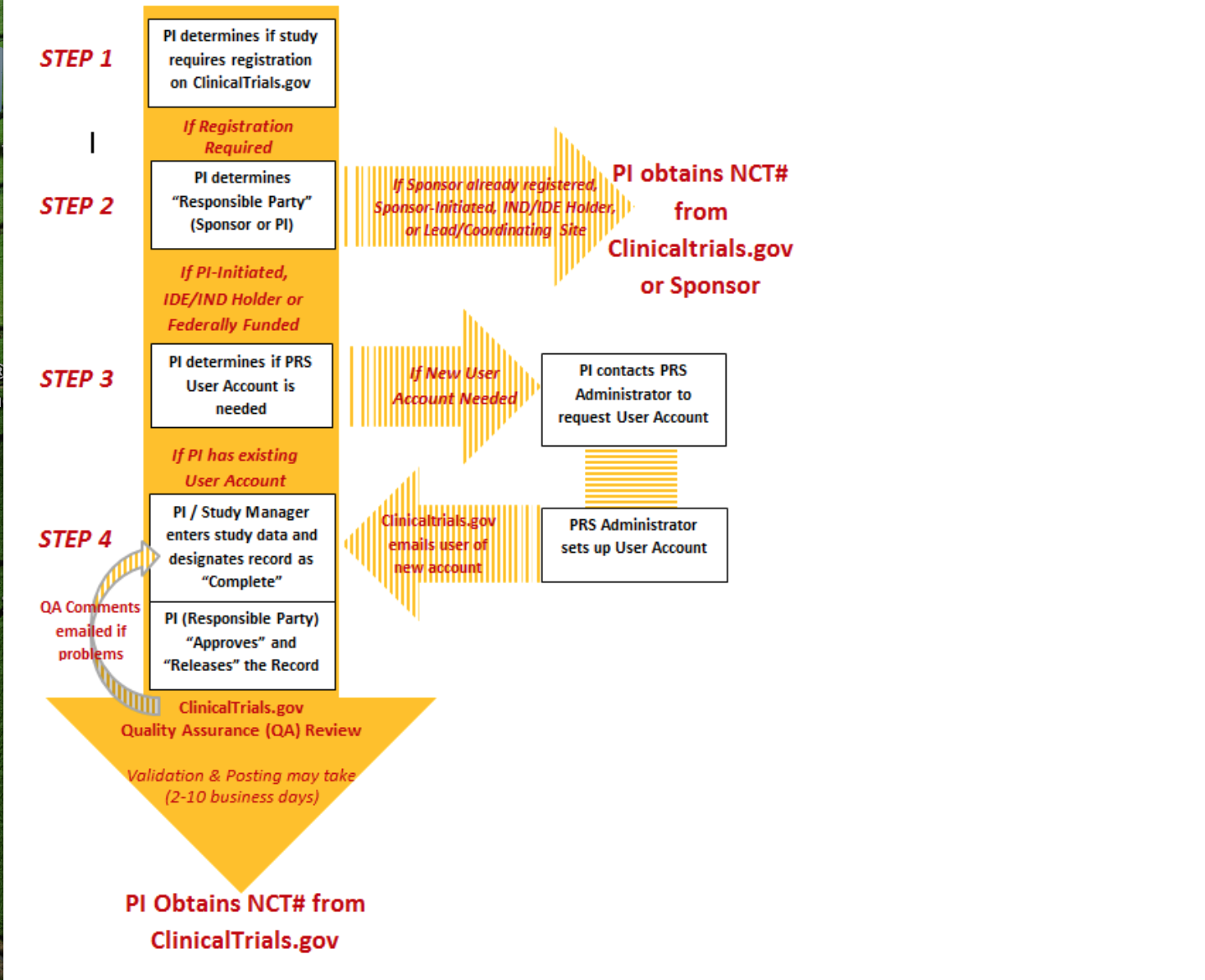
STEPS TO REGISTER STUDY ON CLINICALTRIALS.GOV