SC CTSI leads a USC-wide collaboration to reduce clinical research activation time
A new project is underway to streamline the clinical trial activation process.
Many clinical research faculty and staff can attest to this: starting a clinical trial at USC can be a lengthy, complicated process. A new project is underway to streamline the process and open trials faster.
Select members of the clinical research community began meeting last fall to set a vision, map out current-state processes, and identify opportunities for improvement that will eliminate duplicate steps for a smoother journey for studies from concept stage to patient accrual.
Who is involved?
In his dual roles as Vice Dean for Research in the Keck School of Medicine of USC and Director of the Southern California Clinical and Translational Science Institute (SC CTSI), Dr. Thomas Buchanan is leading the process improvement initiative with the help of all entities involved in trial activation – Institutional Review Board (IRB), Clinical Trials Office (CTO), Department of Contracts & Grants (DCG), Norris Comprehensive Cancer Center Clinical Investigation Support Office, hospital partners, and others – along with advisors from the USC Marshall School of Business. Representatives from these offices joined investigators and research coordinators to form a task force of more than 60 people who met 5-10 times in smaller working groups. Managing the initiative is Allison Orechwa, Director of Programmatic Development at SC CTSI.
What is the vision?
The task force aims to reduce activation time to less than 3 months, eliminate unnecessary or burdensome steps in the process, and increase transparency about the process. There are also plans to create a single portal for study submission and status updates. The ultimate vision is that the Keck School of Medicine of USC and its clinical research enterprise will:
- be viewed by other academic medical centers as a leader in the conduct and support of clinical research;
- be viewed by study sponsors as the place to go for efficient and high-quality clinical trials;
- be viewed by our investigators and staff as efficient, streamlined, collaborative, and user-friendly;
- be viewed by our institutional leaders as a critical component in pursuit of excellence in research, education and health care; and
- promote the conduct of studies that will improve the health of the patients we serve.
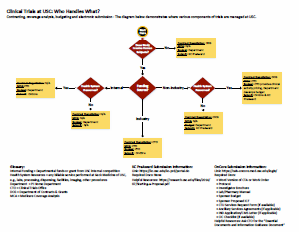
What has the task force produced so far?
In creating process maps for the various phases of study activation, the task force identified a need for clear guidance for study teams on which office handles which phase. In general, CTO handles industry-funded studies, and DCG handles the rest. CTO and DCG sometimes work together on the same study, for example if a federally-funded study will use health system services such as pathology. In that case, CTO will step in to perform the Medicare Coverage Analysis.
More than 50 solutions have been identified to improve processes, such as using external IRBs more often and creating a route for abbreviated review by CTO. The offices involved have committed to implementing the solutions over the next 6-12 months.
When will the process become smoother and faster?
You should begin seeing improvements immediately. For example, ancillary services like pathology and pharmacy are now included early on in the CTO budgeting process, avoiding last minute delays in study start-up. Other improvements require more extensive discussion, for example omitting extra approval steps when budget changes do not exceed a certain threshold.
What is my role/how can I help?
If you are an investigator or part of a study team, recognize that you can help shorten study activation times, as well. Just as the IRB, DCG and CTO work to sharpen their operating procedures, you might examine your own internal procedures. For example, do you always respond to notifications and approval requests immediately, or do they sometimes linger in your inbox? Should you meet with your team more often or log into systems like iStar more regularly? Do you prioritize, to the extent possible, kick-off meetings with the CTO to ensure a mutual understanding of what your study entails? Have you warned your department chair and other approvers that you are preparing a study and would appreciate their quick turn-around when it reaches their inbox?
If you are in an administrative role in CTO, DCG or related office, ask your director about changes that are coming as a result of this initiative. Ask for documentation or reminders to ensure you are following the new procedures and general best practices.
If you are a new investigator, take the time to understand the process and get to know the administrators in the various offices.