Tools for Investigators: USC's Clinical Studies Directory & Research Volunteer Registry Make it Easy for Clinical Researchers and Potential Study Participants to Connect
Recent improvements to the web-based recruitment tool better enable clinical researchers to advertise their studies via social media and other key online platforms.
All clinical investigators know how difficult it can be to recruit study participants, and have seen too many studies fail simply due to lack of participants. But a major upgrade to an important online recruitment tool promises to improve the odds.
To help clinical researchers find participants, the Southern California Clinical and Translational Science Institute (SC CTSI), working with Keck Medicine of USC, launched the Clinical Studies Directory and Research Volunteer Registry five years ago. The SC CTSI recently overhauled the directory to take advantage of social media and other digital platforms. They also redesigned the volunteer registry to make it easier for members of the public to enroll, search and match their health interests with current studies at USC.
With the growth of social media platforms such as Twitter and the public's widespread use of search engines for their information needs, the online and communications world of today is quite different than it was even a few years ago. For researchers, that means potential study participants may already be searching for you. It also means there are more powerful ways than ever to find and connect with potential participants.
According to the Pew Research Center, Americans from virtually every community turn to the internet for the latest health information and science. The Clinical Studies Directory and Research Volunteer Registry takes advantage of platforms such as Twitter, Facebook, Google, and other online or mobile technology to help scientists recruit study participants.
"Clinical research cannot advance without participants and we as investigators must make effective use of these online tools so we can reach the public on the platforms they already use every day," said Katja Reuter, PhD, Director of Digital Innovation and Community at the SC CTSI. "The Clinical Studies Directory has been redesigned to promote your research studies and to take advantage of the internet's unique ability to reach the right audiences.”
Here is what you need to know to list your study in the Clinical Studies Directory and power your access to the Research Volunteer Registry.
Image 1. Create Your Study Page
Clinical researchers seeking participants include their studies in the Clinical Studies Directory by creating a study page. Filling in a simple web-based template, researchers list basic information about their study, including its topic and purpose. Most importantly, researchers include parameters of the type of participants sought, such as gender, age, relevant keywords, and qualifying health or personal attributes. From this template the system will generate what will ultimately become the public and searchable web page for their study.

Image 2. The Public Study Page
Published study pages use lay-friendly descriptions that provide potential participants and their families what they need to know about the proposed research studies. Each page provides brief explanations of the goals of the study, as well as key information such as eligibility requirements, length of the study, compensation, and study site address(es) as well as contact information for the research team conducting the study. The new directory templates now recommend lay-friendly language and terms to help researchers generate the study pages. Research data showed that these lay-friendly formats received 10 times more page visits and sessions, and nearly four times as many contact requests as more technically-worded descriptions. Prior to publication, study pages are reviewed by SC CTSI and IRB representatives.
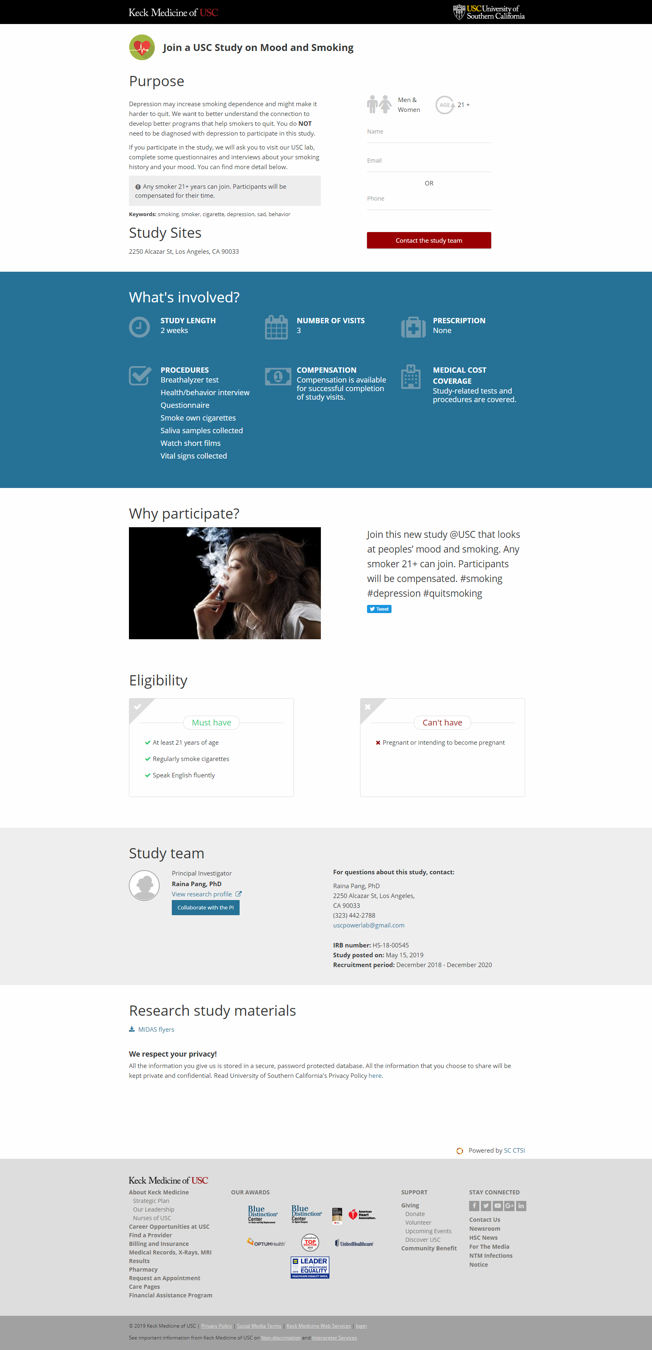
Image 3. Study Page Analytics Dashboard
After a study page has been published in the Clinical Studies Directory, study team members can view the analytics dashboard to view performance metrics including page visits, average time on page, and contact requests. Analysis also breaks down the percentages of traffic driven to study pages from social media platforms such as Facebook, Instagram, Twitter, and Reddit, as well as which search engines drove page visits.
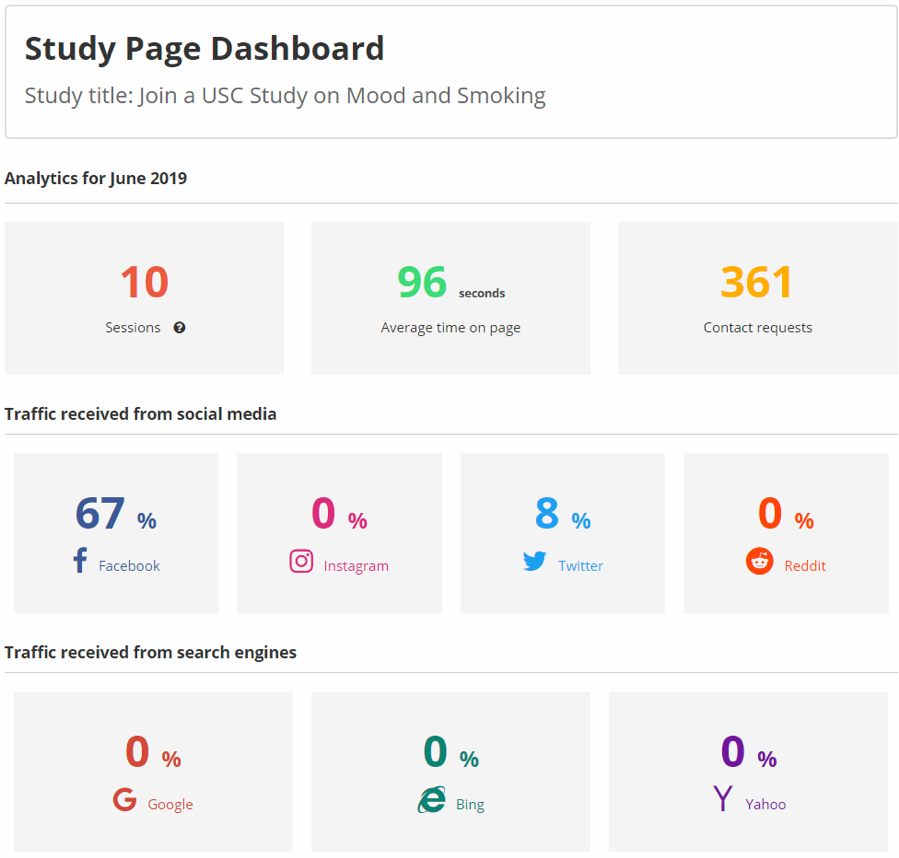
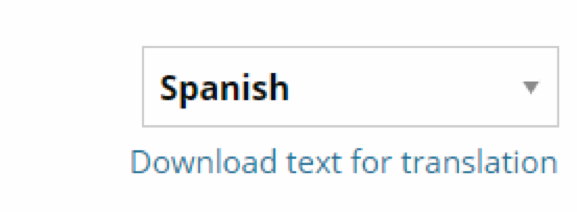
Image 4. Language Translation Tool
Improved engagement of diverse communities in clinical research is fundamental to the mission of the NIH National Center for Advancing Translational Sciences and USC. The Clinical Studies Directory provides support to help translate study pages into Spanish and Chinese to more effectively extend recruitment to those communities.
Next Steps:
Going forward, the SC CTSI and Keck Medicine teams will continue work to enhance the effectiveness of the Clinical Studies Directory. Gaps to be addressed next include:
- Improved tracking of enrollment that was enabled through the Clinical Studies Directory through system-integration with the CTMS and other relevant systems.
- Training opportunities and develop more efficient ways to help study teams develop lay-friendly study pages, including testing effectiveness of tools such as the lay-term recommender and development of new tools, if needed.
- Continue to provide Digital Study Recruitment Support Service for USC and non-USC researchers to help teams recruit study participants via different digital and social media.



