Update on the Clinical Research Harmonization initiative
New analysis reveals faster, more efficient trial activation
New data indicate substantial improvements in the clinical study activation process at USC. Average approval times for the Institutional Review Board and the Clinical Trials Office are down and the clinical research community reports a much better experience in submitting and activating clinical trials.
One driver of change has been the university-wide Clinical Research Harmonization initiative, which set out in 2016 to streamline clinical trial administration at USC, starting with the study activation process. A taskforce of over 60 representatives—including study teams and administrators—mapped out workflows and found many opportunities for improvement within USC’s complex clinical trials system. The result was a set of streamlined process maps which eliminated 27% of the steps formerly required to open a study.
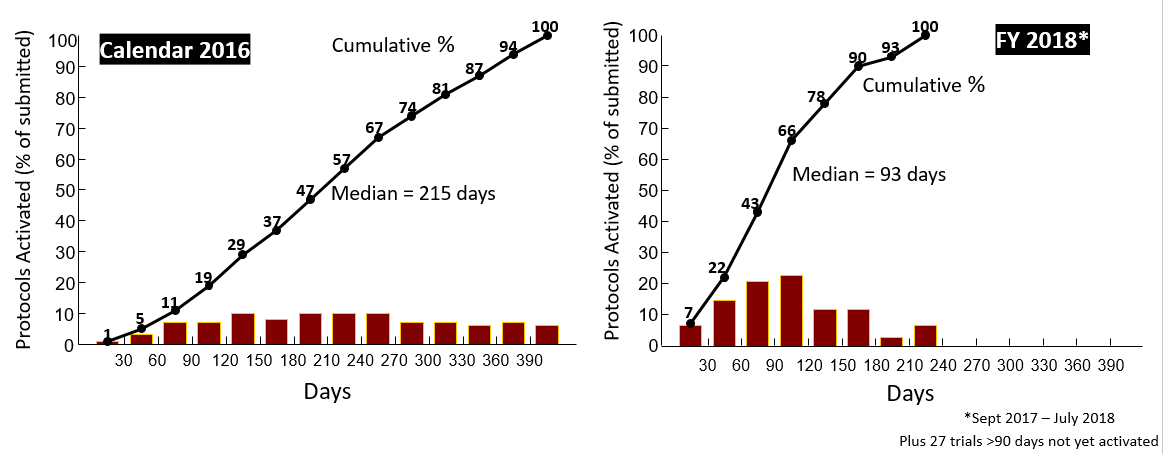
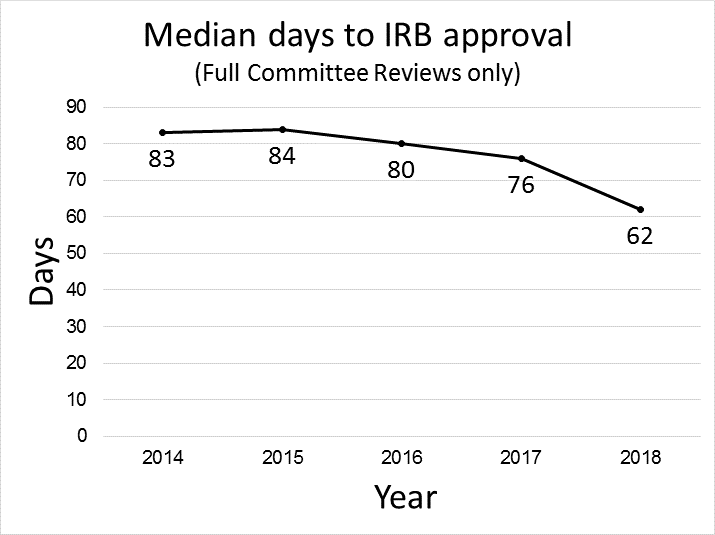
Two years after its inception, the task force has implemented three quarters of the workflow changes, which range from electronic notifications to cost standardization. These and other significant shifts in USC’s infrastructure, such as new CTO leadership and expanded IRB and CTO staffing, have led to tangible results. Compared to 2016, the median time for processing and activation of a trial by CTO has fallen 57%, from 215 days to 93 days. The median time for IRB approval of a full-board clinical trial has fallen 23%, from 80 to 62 days. These results are graphed in the figures below.
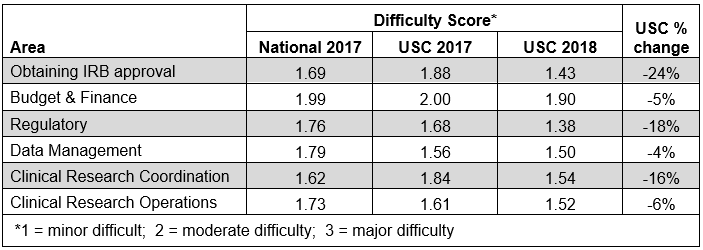
The research community is also feeling better about the process. When asked to rate the level of difficulty experienced in different tasks related to clinical trials, survey respondents reported better average scores compared to a year ago. Improvements were seen across the board, with a 24% decrease in perceived difficulty obtaining IRB approval. Budget and finance tasks are also improved compared to last year, but they remain the among the most challenging aspects of conducting clinical trials at USC.
To make sure the improvements in study activation are sustained, Standard Operating Procedures and other materials have been developed. A guidance document called “Who Handles Budgeting and Contracting” is now available on the Clinical Trials at USC website. It is designed to help study teams submit the right information to the right office. A list of Best Practices (see below) can provide further guidance for successful study start-up. Finally, the Guide to Clinical Research at USC is being updated to reflect the new processes in detail.
Study Team Best Practices for Successful Study Start-Up
- Dedicated staff coordinate study preparation and submission activities in close collaboration with PI/unit director
- Study personnel are identified, trained, credentialed and privileged prior to study activation
- Enrollment feasibility is assessed for each study
- Robust study design is ensured by pre-review of science (e.g., with domain experts, biostatisticians)
- Study documents are submitted to CTO and regulatory bodies (IRB, RSC, Hospital) in parallel
- Submissions and applications are completed thoroughly and accurately using available resources
- Contingencies are addressed right away
- For industry-sponsored studies, SIV scheduling begins before contract execution
Taskforce members
Leon Altman, Daniel Amaya, Melissa Archer, April Armstrong, Zeno Ashai, Grace Barba, David Bateshhansky, Ben Bell, Michael Bowdish, Thomas Buchanan, Yolee Casagrande, Jean Chan, Charlene Chiu, Melody Chun, Kristin Craun, Denise Deack, Bhushan Desai, Vivek Dharne, Michael Dube, Anthony El-Khoueiry, Sheryl Frazier, Suzanne Garcia, Judy Genovese, Manny Gimenez, Susan Groshen, Pat Gutierrez, Yolanda Gutierrez, Richard Hagy, Ben Holstein, Syma Iqbal, Sandy Jean, Nicki Karimipour, Sara Katrdzhyan, Carolyn Keierleber, YoonHee Kim, Quinnie Le, Stacy Lentz, Wendy Mack, Susan McCarthy, Luis Mendez, Melissa Minor, Phillip Moore, Jeri Muniz, Kathleen Oelgart, Joanne Pak, David Peng, Joseph Phillips, Melissa Ramos, Randal Ray, Mike Rice, Maria Elena Rios, Valenta Rodina, George Sarkissian, Amanda Schmitz, Linda Sher, Ira Shulman, Daisy Sosa, Darcy Spicer, Gail Starks, Janet Stoeckert, Esther Suko, Jeannine Taylor, Teresa Trejo, Joyce Tull, Nick Vyas, Megan Watt, Ellen Whalen, Grace Wong.
Facilitators
Allison Orechwa, Nick Vyas and students of the USC Marshall Center for Global Supply Chain Management




