USC Neuroimaging Scientist Awarded NIH Research/Training Grant to Develop Novel Early Marker for AD
The grant enables Lirong Yan, Ph.D., to further develop her noninvasive MRI-based method of assessing the brain's vascular health and supports her advanced training as a clinical investigator.
As the sixth-leading cause of death in the United States, and a major cause of cognitive impairment and dementia, Alzheimer's disease is devastating for millions of patients and their families, and an enormous healthcare burden. It's also exceedingly difficult to treat: by the time symptoms appear, irreversible neuronal degeneration has already taken place, making early detection of the disease a critical element of treatment.
However, a practical method of early detection of Alzheimer's has so far proven elusive, with the search for disease markers largely focused on deposits of amyloid-β plaques in the brain that are believed to play a central role in the disease.
Recently, however, a new direction for early detection has emerged. "A growing body of evidence is suggesting that vascular dysfunction in the brain may precede the cognitive decline and neurodegeneration of Alzheimer's by many years," said Lirong Yan, Ph.D., Assistant Professor of Research Neurology in the Keck School of Medicine of USC.

Lirong Yan, Ph.D
An MRI-based method for measuring vascular health in the brain.
But directly measuring the function of blood vessels, or vascular compliance, inside the brain is not easy. Yan—a researcher at the Laboratory of Neuro Imaging (LONI), of the Stevens Neuroimaging and Informatics Institute—has proposed a noninvasive method of measuring intracranial vascular compliance using magnetic resonance imaging (MRI).
Yan is using a method called dynamic arterial spin labeling to measure intracranial vascular compliance. Previous such approaches have focused on central or peripheral vascular function, but not inside the brain itself, said Yan.
The NIH has awarded Yan a K25 grant to study and develop the arterial spin labeling-based MRI technique to measure vascular brain health. The grant will also support additional training to help Yan develop as a principal investigator in clinical and translational research. K25 grants are career development awards intended to help early-career researchers with quantitative scientific and engineering backgrounds train in and pursue clinical or translational research.
The SC CTSI assisted Yan with both her research studies and her application for the NIH K25 grant. Wendy Mack, Ph.D., Director of Biostatistics at the SC CTSI, is one of Yan's mentors; she helped Yan with study design and statistics. In addition, SC CTSI Career Development Director Cecilia Patino-Sutton, M.D., Ph.D., M.Ed., helped Yan complete her application for the NIH K25 grant, particularly with guidance in the writing of the career development statement that is an important element of the application.
Yan said the expected outcome of her research will be the development of a reliable and noninvasive MRI tool for the assessment of intracranial arterial stiffness, as well as preliminary data about the role of the brain's vascular function in Alzheimer's.
"Ultimately, we hope that arterial spin labeling can detect changes in vascular compliance related to Alzheimer's before extensive neurodegeneration has occurred, when intervention may be more effective," said Yan.
Insufficient vascular compliance is also an important risk factor for other conditions related to the health and function of blood vessels in the brain, including cerebral vascular disease, hypertension, diabetes, and stroke. "So arterial spin labeling can potentially be a marker for all of these kinds of diseases, and a means for earlier detection," said Yan.
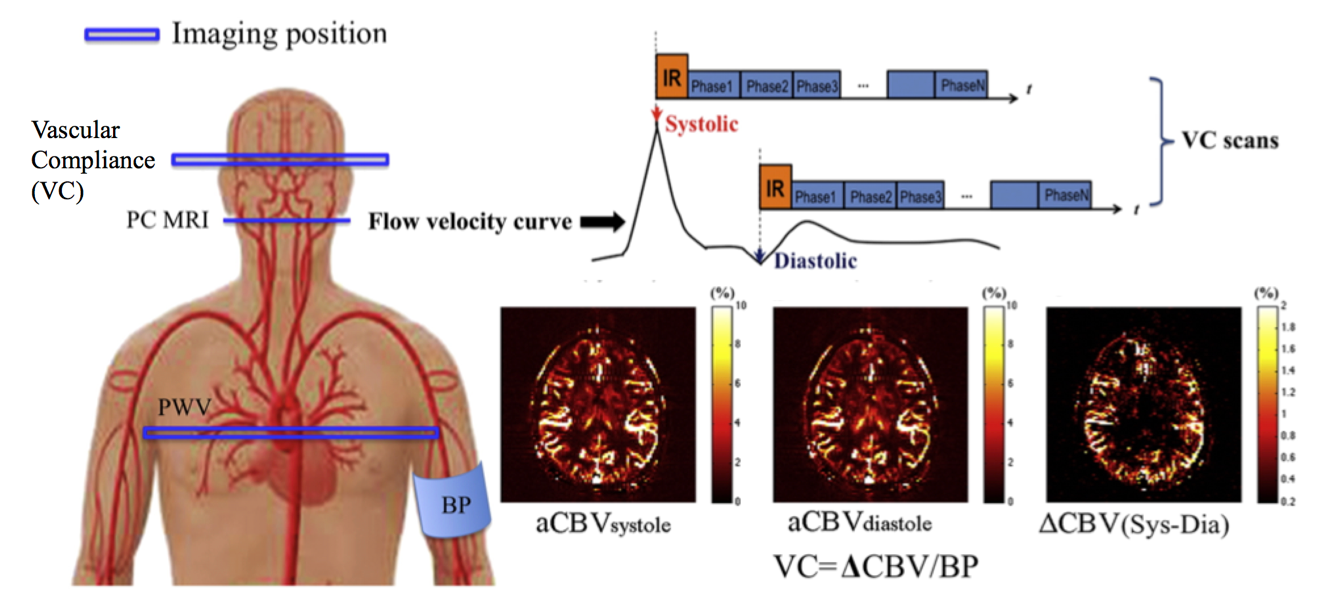
Fig. 1. Illustration of the VC technique applied in the present study. Mean flow velocity curve in the internal carotid artery (ICA) across the cardiac cycle was acquired using PC MRI, by which the delay time at the peak systolic and early diastolic phases was identified. Intracranial VC was assessed by synchronizing dynamic ASL scans with the systolic and diastolic phases of the cardiac cycle using mutil-phase bSSFP scan. Arterial CBV maps at systole and diastole were calculated based on the tracer kinetic model. Intracranial VC was calculated by the changes in arterial CBV between systole and diastole in response to changes in arterial pressure.



