USC/CHLA Investigator Develops Improved Method to Enable Earlier Assessments of Lung Health for Babies with a Positive Newborn Screening for Cystic Fibrosis
A new class of drugs has the potential to add years of robust health for children with cystic fibrosis—if administered at the right time. The noninvasive, improved method established by Danieli B. Salinas, MD, can spot early signs of lung disease in newborn babies to guide diagnosis and care.
Thousands of children are born each year with genetic variants associated with cystic fibrosis (CF), a disease that can interfere with function of the lungs and pancreas, leading to a range of resulting health impacts and possibly to lifespans shortened by decades.
Fortunately, new medicines called modulators can improve the function of the defective protein that causes the damage in children with CF, slowing the progress of the disease and diminishing its impacts on health. But there are more than 2,000 CF gene variants: some don't cause disease at all, others express as a spectrum from mild to severe, and most are still a mystery to researchers.

As a result, CF specialists now place an increased emphasis on detection and treatment of the earliest signs of lung disease in children, explained CF researcher Danieli B. Salinas, MD, MS, an Associate Professor of Pediatrics at the USC Keck School of Medicine practicing at Children’s Hospital Los Angeles in the Pediatric Pulmonology Division. Salinas is also an alum of the Southern California Clinical and Translational Science Institute's KL2 Mentored Career Development program.
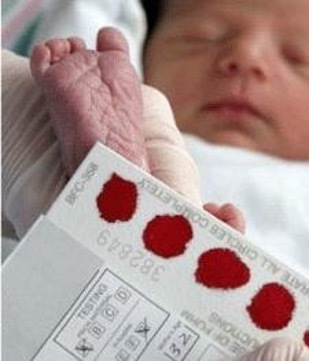
Newborn babies in the U.S. are all blood-tested for CF genes, but even those with severe CF mutations tend to have healthy lungs at birth. This gives doctors armed with the modulators and other strategies a valuable window for intervention—if they can figure out which CF babies are actually at risk for developing serious disease.
"You can save the health of the lungs through early treatment," said Salinas. "You don't want to wait until the kids have scarred lungs."
For several years, Salinas’ research has focused on developing better ways to assess the health of CF children, to help inform both diagnosis and treatment. Recently, she published two papers describing her lab's advances in developing methods to detect early CF disease in children who had a positive CF newborn screening. These innovative tests can be used in infants and preschoolers, both providing important advantages over existing standard of care methods.
The current standard in confirming the diagnosis of CF after a positive newborn screening is the sweat chloride test, which measures the concentration of chloride in collected sweat, an indirect way to estimate the function of the CF protein. The sweat chloride test can identify severe cases, but is insufficiently sensitive to identify mild to moderate CF disease.
By measuring the sweat secretion rate, instead of the concentration of chloride in collected sweat, one can directly measure the CF protein function. However, methods measuring sweat rate developed so far are complex and time consuming, requiring patients to stay still for hours. Salinas envisioned a new approach to the sweat rate test that uses digital imaging technology and can be completed in 20 minutes, developed over the course of four years in collaboration with biomedical engineers at the USC Viterbi School of Engineering. Their approach has proved to be a more sophisticated way to measure sweat rate, and has the advantage that it can be used in infants.
Salinas is currently working on a human nasal epithelial cells assay able to precisely measure CFTR function at an individual level.
"The estimation of risk is really at the heart of my research," said Salinas. "I'm hoping that these two tests (human nasal cells and the sweat rate) will lead to a simple, definitive answer for newborns and their families. These tests will be able to tell us the risk of developing CF and estimate severity of disease. They will serve as diagnostic and prognostic tests and will guide care by defining potential responses of patients to the various CF protein modulating drugs," she said.
Four modulators are currently approved, with two others in the pipeline. Detecting early signs of lung disease is similarly important for preschool-aged children with CF genes whose health is still good, and for whom early medical intervention can increase the odds of normal health through childhood. Salinas showed that a simple Lung Clearance Index test can detect early signs of lung disease by measuring nitrogen washout during normal breathing. The method is easier to administer to preschool children than other diagnostic methods, and just as sensitive as a chest CT scan, providing doctors with important prognostic information about these young patients, noninvasively.
Salinas said the mentorship and training she received in the SC CTSI's KL2 researcher training program provided an important boost to her transition into clinical research. Of all the benefits of the KL2 program, however, Salinas said the most important was how it helped her build relationships with other clinical investigators.
"My background prior to coming to USC and CHLA was in basic science research," she said. "I liked the bench and was good at it, but when I switched gears to clinical research to come here, I missed the contact with investigators that I had before. The KL2 was a bridge for me to build relationships with other USC investigators, including the biomedical engineers at Viterbi."
The KL2 support and protected research time enabled Salinas to build her research program. SC CTSI mentors helped her apply for other support, and she is currently funded by the Cystic Fibrosis Foundation's Therapeutic Development Network Clinical Research Scholar Program, and also has a fellow who is fully funded.
"I couldn't do what I am doing now without the support I received from the SC CTSI," she said.
Salinas' paper, "Image-based β-adrenergic sweat rate assay captures minimal cystic fibrosis transmembrane conductance regulator function" was published recently in the journal Pediatric Research. Her article "Abnormal Lung Clearance Index in Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID) Children with Otherwise Normal FEV1.," was published in the journal Lung.



