How the Trial Innovation Network Helped this Investigator Redesign her Study Proposal to do More Science with Less Money
Following NIH review, University of California, Irvine, researcher Katrine Whiteson needed to resubmit a proposal that met requests to expand the study, but to manage it on a significantly reduced budget. Advisors in the Trial Innovation Network, a researcher-support program of the National Center for Advancing Translational Sciences, helped her solve the problem.
"It felt like an impossible situation," said Katrine Whiteson, PhD, who studies the human microbiome at University of California, Irvine, where she is associate director of UCI's Microbiome Initiative, and assistant professor of Molecular Biology and Biochemistry, and of Pediatrics. "The reviewers asked for more samples, but at the same time the budget in the RFA was reduced to $3 million over four years, from $5 million over five years."
"We felt really stuck."

Whiteson was describing an experience that is likely familiar to clinical and translational investigators. Her proposal to study the human gut microbiome, submitted in response to a request for proposals from the National Center for Advancing Translational Sciences, was not immediately funded, but it was well-received by reviewers, who gave it a strong score. As is typical, the reviewers' critique called for additional data collection and analyses. But then the updated RFP was released, with its decreased time and funding, and it was not at all clear if the study could go forward.
Whiteson and her team turned for help to the Trial Innovation Network. The NIH recently created the Trial Innovation Network to accelerate and improve multicenter translational research by streamlining administrative tasks and by making senior researchers available for advice and guidance, among other measures. The network comprises three partners: Trial Innovation Centers, Recruitment Innovation Center, and the 50-plus CTSA Program hubs across the country. (See this SC CTSI article for more information.)
The study of the human microbiome has expanded considerably in the last decade as scientists and funders increasingly appreciate the important roles these microbe populations play in human health and sickness. Whiteson's proposed study on the newborn and early life microbiome would leverage a rich source of data: the medical records collected by Kaiser Permanente's many Southern California medical centers. Kaiser Permanente cares for more than 9 million Californians, about half of them in Southern California, and its patient medical records represent immense scientific and research value for investigators.
"That data could keep us busy for decades because people stay with Kaiser Permanente for decades," said Whiteson. Her team hoped to study the gut microbiomes in 1,000 of the 40,000 babies born in Southern California Kaiser Permanente sites each year. "It is a great opportunity to study a robust sample from an ethnically diverse population that really represents Southern California," said Whiteson.
The study would also support one of NCATS' central goals: the creation of systems that enable clinical and translational researchers to more efficiently study the data regularly collected by major health providers.
At the suggestion of Dan Cooper, MD, the director of UCI’s Institute for Clinical & Translational Science, Whiteson applied to the Trial Innovation Network. Whiteson had received her scores and comments from the NIH in April, and a resubmission deadline of July, so time was short. Her proposal request for trial design assistance was approved by early May, and she was quickly contacted by Cecile Santiago-Turla, MD, MHSc, project leaders at Duke Clinical Research Institute, who facilitated interactions with several sub-groups within the TIN.
Whiteson and her colleagues began speaking with researchers at various institutions around the country who were part of the Trial Innovation Centers and the Recruitment Innovation Center. "We had tons of conference calls and it was extremely helpful to have these people to bounce ideas off of," she said.
But a few of the ideas may have made all the difference. One came from Frank Rockhold, PhD, a professor of biostatistics and bioinformatics at Duke University. "He had the great suggestion to randomize the subjects into sub-groups and take different time-points from each, allowing us to explore time-point space with fewer samples overall and fit within the budget," said Whiteson. "That was the moment I felt we had a solution to this really hard problem.
Other Trial Innovation Network advisors also provided key suggestions. "We got great advice from the Recruitment Innovation Center about how to clarify our recruitment plan in the grant, and make sure that we got help from Community Engagement Studios and worked to share the results of the study with the community to maximize the benefits of the study for participants and researchers, alike," said Whiteson. "It was truly helpful to have had the support of this group of people who are extremely skilled and experienced."
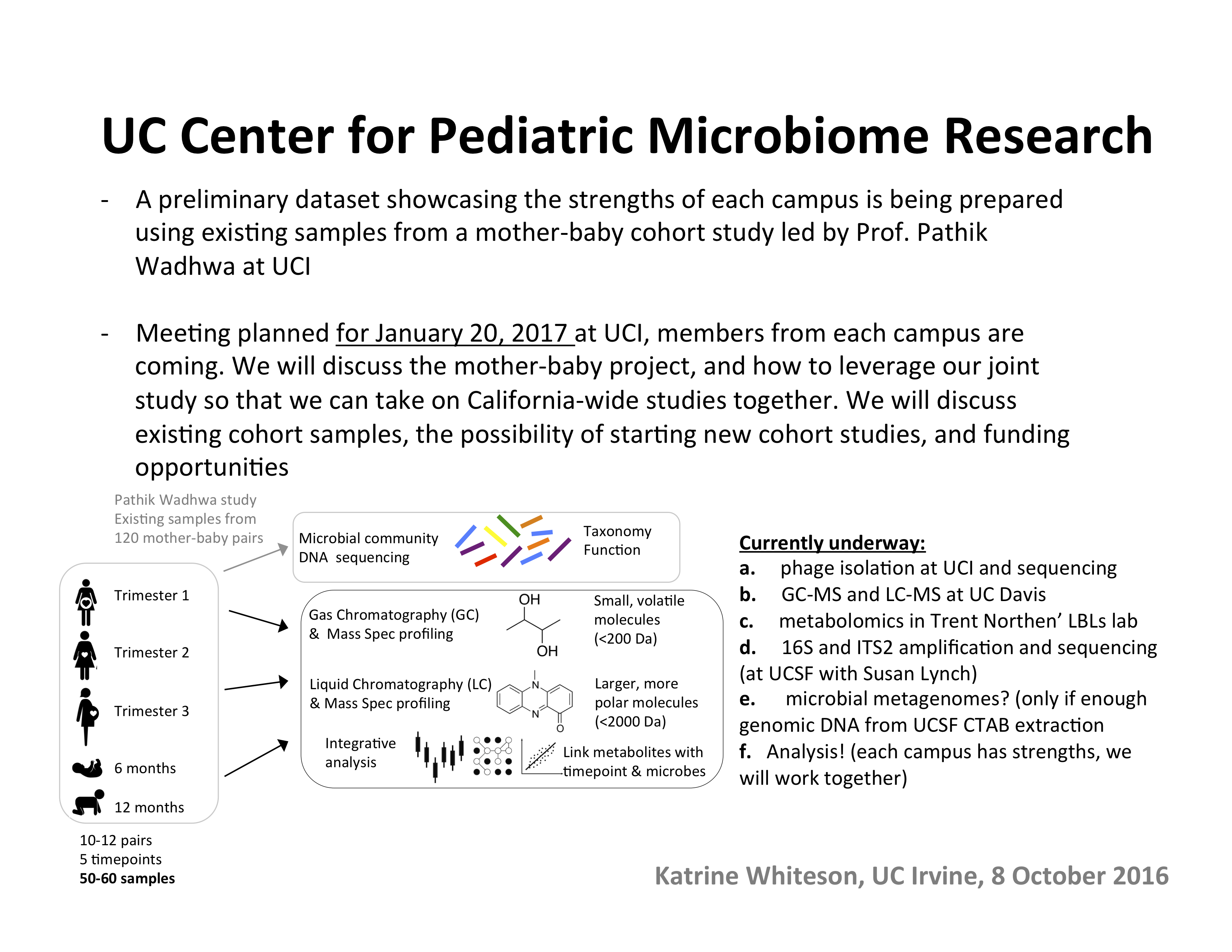
With the assistance from the Trial Innovation Network, Whiteson's team was able to redesign and resubmit their study proposal in July 2019. They now await a final response on funding, but are confident they had successfully addressed the concerns and requests raised in the initial review.
Researchers in the USC and CHLA research community can connect with the Trial Innovation Network through the Southern California Clinical and Translational Science Institute. Interested researchers should contact USC’s Trial Innovation Network Administrator Nicki Karimipour, PhD, at 323-442-1280 or Nicki.Karimipour@med.usc.edu.



