NIH Launches Trial Innovation Network to Streamline and Improve Multicenter Research
The SC CTSI and all CTSA hubs across the nation are part of the new Trial Innovation Network. The SC CTSI invites USC and CHLA investigators to learn how the program can simplify IRB applications, support patient recruitment, and provide other assistance.
Although multicenter studies have grown in both importance and number in recent years, regulatory approval and other key aspects of the research process remain as time-consuming as ever—and can even derail potentially valuable studies.
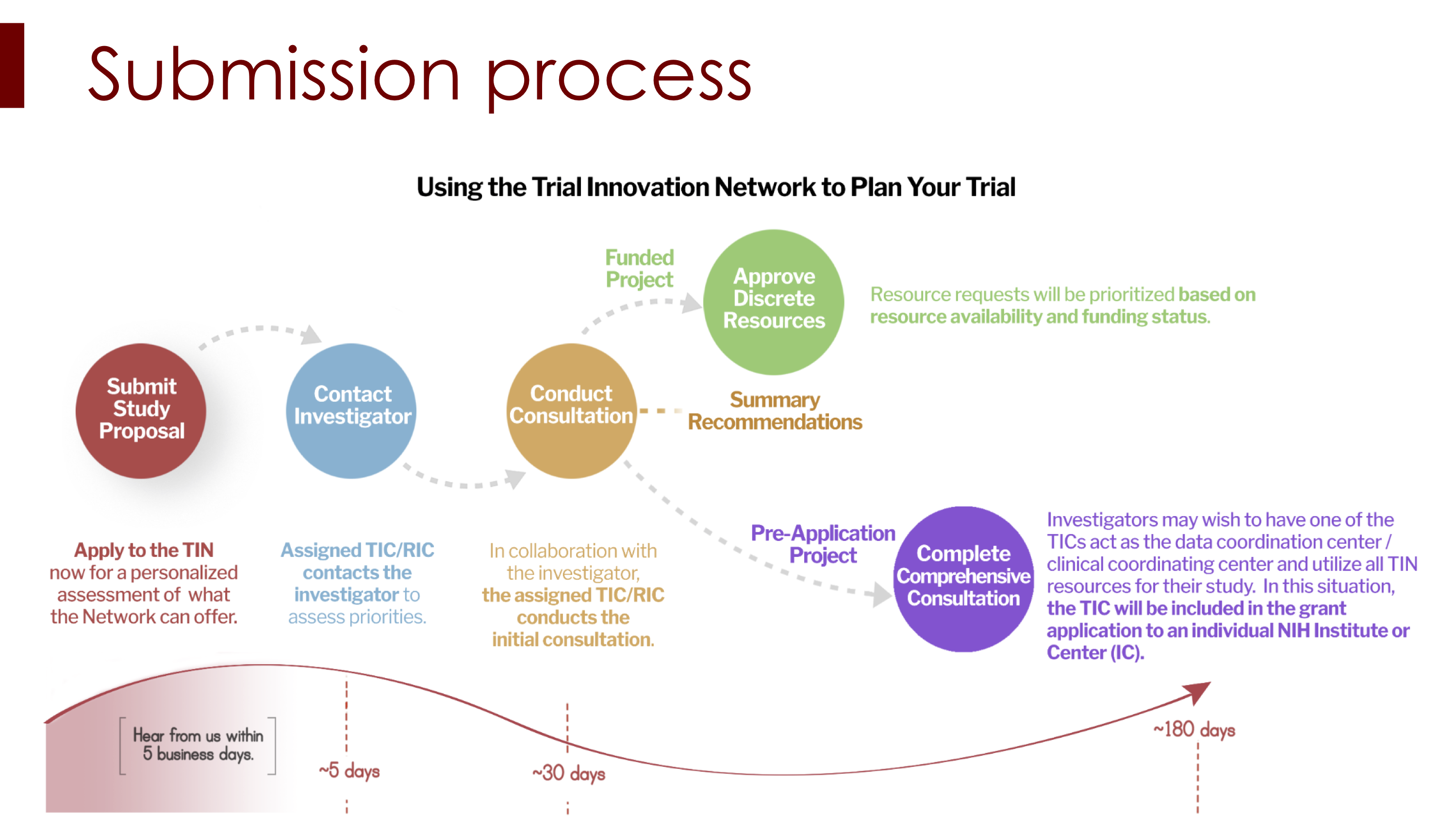
To accelerate, simplify and improve the entire process of multicenter clinical and translational research, the National Institutes of Health recently established the Trial Innovation Network (TIN), a collaborative initiative within the National Center for Advancing Translational Studies award program.
The TIN is composed of three key organizational partners: the Trial Innovation Centers, the Recruitment Innovation Center, and the 50-plus CTSA Program hubs located at medical research centers across the country.
At the Southern California Clinical and Translational Science Institute, the TIN is administered by the Clinical Research Support team. Investigators developing multisite studies can obtain assistance on a number of fronts, explained April Armstrong, MD, MPH, Director of Clinical Research Support at SC CTSI.
"We can work with the Trial Innovation Network to help with issues like contracting and budgeting, as well as with specialized support for recruitment," said Armstrong. "And for investigators who are thinking about designing such studies, the SC CTSI can also provide unique expertise to ensure that the multicenter studies are well-designed to address their specific research questions."
The TIN's areas of assistance include:
- A central, single Institutional Review Board (SIRB) process to organize and harmonize the IRB requirements and reviews across all of the institutions and stakeholders involved in multisite studies, saving time and costs.
- Standard contracting and budgeting language templates to simplify and streamline what are frequently major obstacles for principal investigators initiating multisite studies.
- Recruitment assistance to help ensure success in what is frequently one of the most challenging aspects of clinical and translational research.
“The multicenter clinical trial is the essential tool in the development of new therapies, but it is an exceedingly complex, arduous task," said Paul Stephen Aisen, MD, Director of the Alzheimer's Therapeutic Research Institute, a consortium that works with investigators at research centers in Southern California and around the world. "Collaborative nationwide resources, particularly for recruitment challenges, play an invaluable role in supporting these trials.”
Research most appropriate for the TIN program are multicenter studies with more than three sites, which include cross-disciplinary elements. TIN funds proposals from various sources, including NIH Institutes and Centers (ICs) such as NHLBI and NIA; PCORI, foundations and other non-profit organizations, and existing cooperative disease research networks.
In addition, the SC CTSI can provide a number of specific, free, localized resources to principal investigators who initiate multicenter studies through TIN. These include eight hours of biostatistics consultation on research and statistical design, budget preparation support, assistance with submission of regulatory documents, recruitment strategies, design of print materials and messaging suitable for distribution through social media, advice about ad purchasing for digital recruitment methods, among others.
Principal investigators initiating multisite studies can submit proposals to the TIN, which will send out a notice of opportunities for studies seeking to add sites, said Nicki Karimipour, PhD, Project Manager and Administrator for the TIN at USC.
"Overall, the value of the TIN is that it makes the process of multicenter study start-up and conduct faster and more efficient, and it’s easier to identify potential collaborative study sites by leveraging the network of CTSAs around the country," said Karimipour.
Karimipour works with representatives from the Institutional Review Board and the Departments of Contracts and Grants, as well as members from Children’s Hospital Los Angeles for pediatrics trials. Whenever there is an incoming trial available through the Trial Innovation Network, part of her role is to disseminate that information to local investigators at USC and CHLA who may be a good fit to serve as an investigator. To ensure a sufficient number of participants for the trial, the TIN team also runs queries with our electronic medical records.
Armstrong urged researchers to contact CRS at the SC CTSI to learn more about the various services and resources available through the TIN. "Multicenter studies are the core of the Trial Innovation Network, and we're here to help support this kind of research," she said.



