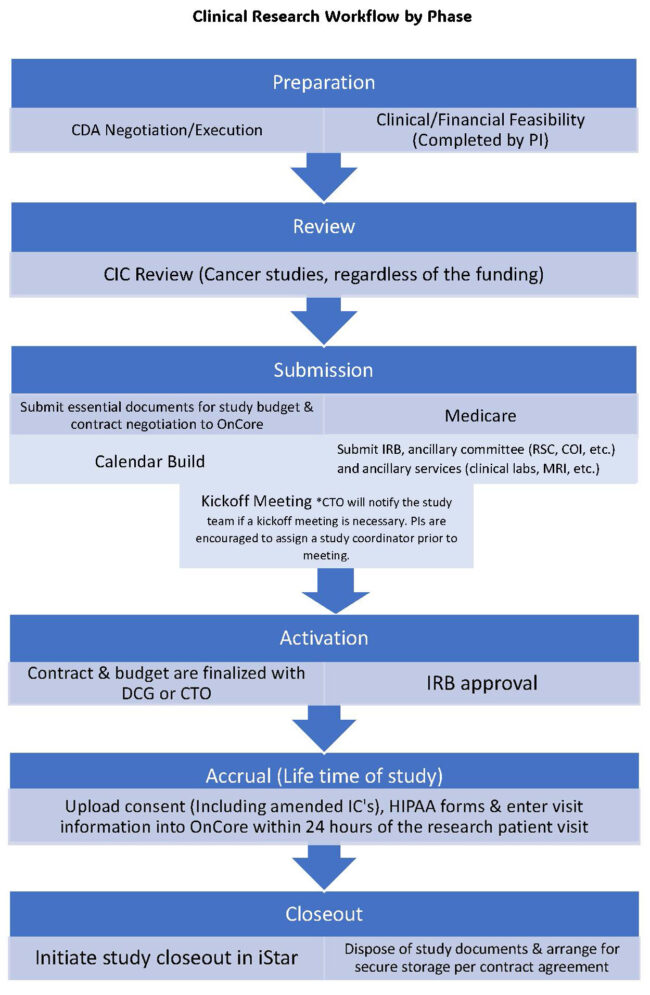
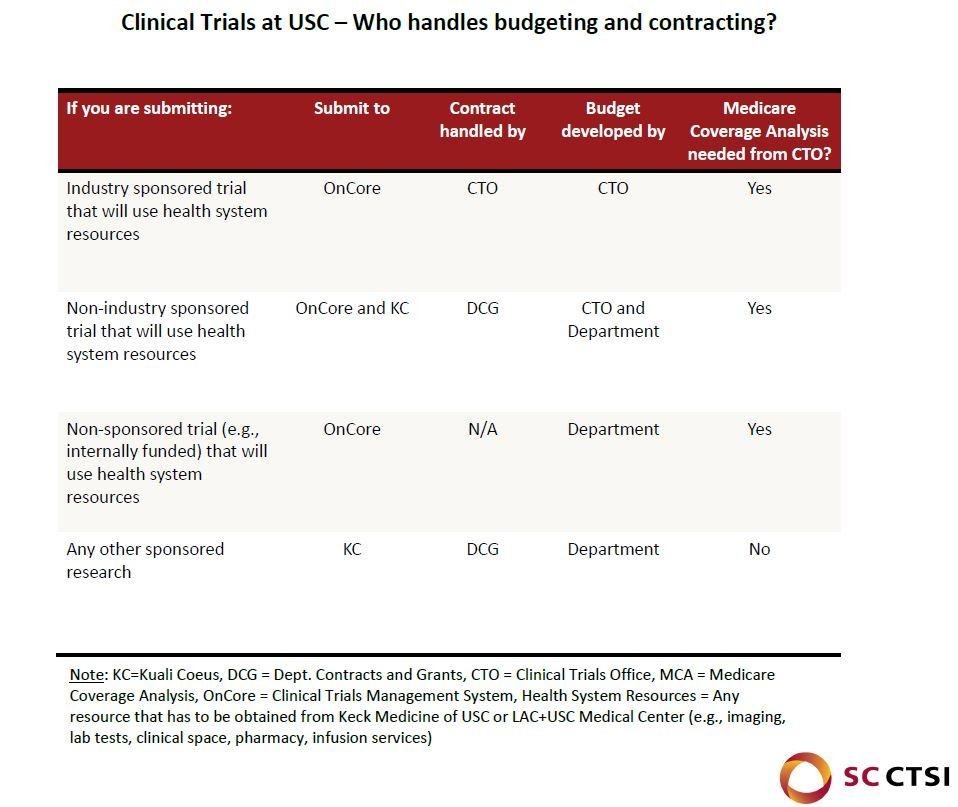
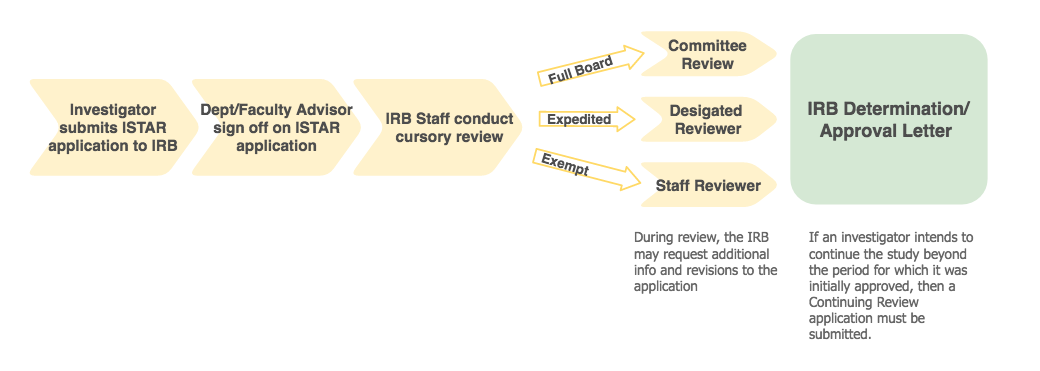
Coordinating ancillary services early on is crucial to ensuring there are minimal delays in the study preparation process. Relevant ancillary services include radiology & imaging, pharmacy, pathology, cardiology, and ophthalmology. There are over 60 research laboratories and services located at UPC, HSC, and CHLA, offering researchers access to state-of-the-art facilities, technologies, resources and expertise across academic disciplines and clinical specialties. Researchers can use the link above to segment searches for labs and services based on location or research area.
The following section provides a description of the services provided, how to request the service, and contact info.
1. USC Medical Plaza Pharmacy
There is another pharmacy, in addition to IDS pharmacy, located on the HSC campus. The Plaza Pharmacy is located in HC2 and can assist with filling prescriptions and dispensing drugs for studies on a limited basis. PIs should discuss needs early on to see if Plaza can accommodate the study drug.
Plaza Pharmacy: Contact Rena Charchian; Director of the Plaza Pharmacy; 323-442-5770
2. Radiology
Radiology and imaging services are available at the Norris Cancer Center and at Keck Hospital (Healthcare Consultation Center II and the PET Center in Healthcare Consultation Center I). Patients recruited from LAC+USC may be imaged at HCC2 or the PET center only if funding is available. The process can be initiated while the study is under review by the IRB. However, no research scans may be scheduled until the study has final IRB approval, which is contingent on RSC approval. In addition, research scans can’t be scheduled until ROF is available and Radiology Scan Request form (if applicable) is completed. Radiology research imaging charges are determined by the coverage analysis performed by the CTO. Please note there is a charge by the Department of Radiology for completion of RECIST tumor flow sheet form by a radiologist. Please make sure CTO is aware and prepares your budget accordingly.
Radiology: The coordinator will send an email to Bhushan Desai (bhushand@usc.edu; 323-865-9949) or contact the Department of Radiology at 323-442-8541, outlining the imaging requirements for the study and provide any additional materials (i.e., imaging manual). Imaging can be requested using the Research Order From created by the Clinical Trials Office.
3. Imaging
i. Center for Image Acquisition
The Center for Image Acquisition, part of the USC Mark and Mary Stevens Neuroimaging and Informatics Institute, houses MRI equipment used for neuroimaging. The Center has other equipment to help PIs conduct scans that ensure the comfort for participants. Click here for a full list of equipment and capabilities.
CRCs can schedule appointments with the Center by creating an account under the “For Investigators” tab. The following information will be needed for the application: project name, proposal number, PI name, anticipated start date, copy of IRB approval letter, copy of study budget showing scan costs. Once the application is approved, login credentials will be provided, and users will be able to schedule online. Investigators preparing budgets for new proposals should contact the center to obtain the latest rates for scans and other services. Please allow at least 2 weeks for rate requests to be approved.
CIA: Direct any questions to CIA@ini.usc.edu.
ii. Molecular Imaging Center – Radiochemistry/Cyclotron Labs
The Molecular Imaging Center (MIC) is a comprehensive imaging program focused on the development of various types of PET tracers used for imaging disease. The facility includes a Cyclotron GMP facility, PET radiochemistry laboratories, access to small animal imaging, and access to clinical PET/CT imaging facilities.
PIs interested in utilizing these services should contact Peter S. Conti, MD, PhD to set up an initial meeting to discuss the feasibility of the study, the radiotracer availability/preparation and clinical trial enrollment. Clinical imaging studies will be performed under pre-IND, IND, or approved drug protocols at HCC-I. Opportunities for early-phase clinical trials and multi-center trials are available at MIC including existing trials in the following areas:
Oncology:
● Breast Cancer
● Prostate Cancer
Neurodegenerative Disease:
● Alzheimer’s Disease
● Parkinson’s Disease
Cardiovascular Disease:
● Cardiac Ischemia
MIC: Peter S. Conti, MD, PhD. 323-442-3858 / peter.conti@med.usc.edu and copy miclab@usc.edu.
4. Cardiology & CardioVascular Thoracic Institute
Echocardiography services are available at the CardioVascular Thoracic Institute (CVTI), Keck Hospital of USC, and LAC+USC Medical Center. The Echo Core Lab can accommodate orders that are more complex.
The CVTI can accommodate outpatient research-related cardiovascular tests such as echocardiograms, electrocardiograms, or vascular ultrasound. As part of each trial, the CTO will develop a standard ROF that includes an echocardiogram request. That form should be submitted to CVTI at the time of research echo request.
For inpatient researcg, echocardiograms can be ordered through Cerner like a regular echocardiogram. In the comments section for the order, input "research" and specify which study so that techs know how to modify echo as needed. If the study would be done for clinical purposes and is not being done for research purposes only, the comment is not necessary.
If the study calls for a regular echo, then staff will do a regular echo. If more complex echos are needed, details will need to be provided in order to educate staff in advance.
How to request service: The PI or study team should contact one of the individuals listed below during the planning process in collaboration with IRB and CTO. The PI or study team will provide protocol, examination details and proposed CPT codes related to the type of echocardiogram needed to fulfill the study and budgetary requirements. The study team should ensure that CTO budgets adequately for the echocardiogram and develops an appropriate research order form. Information on expenses can be accessed via the USC CTO.
The same process for incorporating research echocardiograms into the budget and research order forms applies to studies performed at LAC+USC Medical Center. The PI and study team should be aware that properly budgeted research echocardiograms can be performed at CVTI or Keck Hospital as long as a research order form is completed. If the echocardiograms must be performed at LAC+USC Medical Center and will require a large number of studies on a regular basis, please contact CRU to discuss.
If an inpatient echocardiogram is ordered for research purposes, it will usually be done like any other inpatient echocardiogram. If the echo is urgent (i.e., must be done within 24 hours), then a phone call should be made to the echo lab to make sure it gets done when needed.
CVTI: Clinic Manager Elizabeth Vela elizabeth.vela@med.usc.edu. Click here for additional CVTI staff and faculty contact information.
i. Echo Core Lab
In addition to the standard echo orders and reads, another option for cardiology services is the Echo Core Lab. This resource is available for researchers who require echo reads that require measurements that are particularly complex and outside of the standard echo report. The Echo Core lab is experienced in reading echos for large multicenter trials to ensure standardization and quality. They are available to serve as the echo core lab for IITs and industry sponsored trials conducted at USC.
How to request service: Click here for additional contact information for USC Cardiovascular Medicine
CRU: USC Cardiovascular Research Unit (CRU)
323-442-6863 / fax: 323-442-7610
cru@med.usc.edu.
5. Ophthalmology
The Ophthalmology Clinical Trials Unit at the USC Roski Eye Institute conduct a wide range of clinical trials through a variety of subspecialty services, providing comprehensive ophthalmic exams and diagnostics. Subspecialties include: Cornea and External Diseases, Glaucoma, Oculo-facial Plastic Surgery, Neuro-Ophthalmology, Ocular Oncology, Uveitis and Ocular Inflammation, Retina, Vitreous, and Macular Diseases.
Each ophthalmic examination conducted by the Ophthalmology services will include a broad spectrum of ophthalmic evaluations. The exams may vary depending on the specifics of each clinical trial. A typical exam may include the following: Patient history, visual acuity, depth perception, color vision, eye muscle movements, peripheral vision, pupil response, refraction, tonometry (eye pressure), external examination (cornea, eyelids, conjunctiva), retinal examination/funduscopy (retina, optic disk, macula). The USC Roski Eye Institute offers the most advanced diagnostic technologies through their on-site services. For more information about the wide-range of diagnostic testing that may be conducted in each clinical trial, see below.
How to request services: Ophthalmology service for clinical trials may be accessed through the following procedure:
1. The requesting department PI or CRC should initiate contact with the ophthalmology clinical trial unit by calling 323-865-6935 or by completing our online contact form. For more information on ophthalmic clinical trials click here.
2. The scope of the trial and needed services should be presented through a Research Order Form (ROF), schedule of visits, and instructional documentation related to the requested services.
3. Charges should be obtained through the ophthalmology charge master on file with the CTO.
4. A full review by the ophthalmology PI and ophthalmology clinical trial unit will be conducted with feedback on available resources and clinical registration procedures.
Click here for contact information for the Department of Ophthalmology.
Ophthalmology CTU:
323-442-6335 / fax 323-442-6496 or contact a study coordinator directly
Mariana Edwards
Research Coordinator
USC Roski Eye Institute
Keck Medicine of USC
323-442-6490
mariana.edwards@med.usc.edu
6. Neurodiagnostic Department of USC Comprehensive Epilepsy Center
The USC Comprehensive Epilepsy Center is designated as a level 4 epilepsy center, indicating that the center and its associated staff can provide care for the most complex cases. The Neurodiagnostic Department can provide services such as electroencephalography (EEG). Specifically, routine, sleep-deprived or video EEGs are available for the purposes of diagnosing epilepsy or brain abnormalities. For more information and to coordinate services, reach out to the department directly.
Neurodiagnostic Department:
https://epilepsy.keckmedicine....
7. USC Sleep Disorders Center
The USC Sleep Disorders Center provides inpatient and outpatient sleep disorder evaluations. In addition to providing treatment for a variety of sleep disorders, the center also can be used as a location to conduct sleep assessments as part of a research study. The center includes a four-bed sleep lab and is staffed by board-certified sleep specialists and registered polysomnography technologists. For more information and to coordinate services, reach out to the center directly.
Sleep Disorders Center: sleepcenter@med.usc.edu
8. Pulmonary Diagnostic Services Lab
The Pulmonary Diagnostic Services Lab provides assessments that test pulmonary function, such as lung function evaluation, supplemental oxygen evaluation and bronchospasms evaluation. For more information and to coordinate services, reach out to the lab directly.
Pulmonary Diagnostic Services Lab: KeckPFTLab@med.usc.edu
9. Dermatology
The providers at USC Dermatology possess clinical expertise in the management of rare and common skin diseases like psoriasis, atopic dermatitis, skin cancer, infectious diseases, autoimmune diseases and immunobullous diseases. Clinical dermatologic services that pertain to research patients can include skin biopsies, cyst removal and Mohs surgery. For more information and to coordinate services, reach out to the department directly.
Dermatology: dermatology@med.usc.edu
10. USC Clinical Trials Unit
The Clinical Trials Unit at USC (CTU) provides the infrastructure, resources, and support to conduct human studies, with a focus on early phase and complex mechanistic trials. CTU can assist study teams with accommodating patient visits for high intensity trials that involve serial blood draws, infusions, and monitoring. The CTU also has trained clinical staff like research nurses, phlebotomists, radiology technicians, and more. The staff is highly experienced in collecting and processing PK and PD samples in a timely and efficient manner.
CTU: Yolanda Gutierrez, Assistant Director for Clinical Research Operations; 323-865-3056; cerda@med.usc.edu or ctu@sc-ctsi.org
The CTU also offers a specimen-processing center that offers a variety of services and serves as a one-stop unit for processing, storing, and shipping research samples collected from research patients. The USC CTU has the capability to provide X-ray absorptiometry (DEXA) for body composition or bone densitometry studies. The CTU also provides specimen collection and processing services that may include: blood, saliva, sputum, urine, stool, hair follicles and other types of specimens. In addition, the CTU Processing Center can perform specialized processing for studies in which the assays are run in the investigator’s lab, research core lab, or sponsor’s central lab. Specimen processing may include the following: Serum/plasma separation, buffy coat separation, custom labeling, centrifuging, aliquoting, blood smear, PBMC separation; assistance with shipping & handling (ambient, refrigerated, dry ice). To learn about CTU’s full capabilities, click here.
CTU Laboratory: Lilit Baronikian, CTU Lab Supervisor, 323-865-3379 (office) or 818-642-4838 (cell), yegiyant@med.usc.edu.
11. Diabetes & Obesity Research Institute
The USC Diabetes & Obesity Research Institute (DORI) is a multi-disciplinary collaborative of investigators seeking to link basic, clinical, and public health research to advance the understanding of obesity and its relationship to type II diabetes. DORI operates a clinical research facility in the Clinical Sciences Center that supports outpatient studies of low to moderate intensity, such as interviews, medical histories and physical exams, single and multiple blood draws, and oral glucose tolerance tests. Protocols require participation by study teams; additional research nursing and phlebotomy assistance is available through DORI. Special capabilities include dual energy X-ray absorptiometry (DEXA) for body composition or bone densitometry studies performed by an experienced DEXA technician and a core lab that can be used by study teams to perform sample processing following training and approval by DORI staff members, and a metabolic kitchen and gym for nutritional and exercise interventions and assessments. DORI also has a Nutrition Data System for Research (NDSR) program that is used for dietary analysis and available to share with other PIs. Facilities and services are provided at a cost to PIs and their study teams.
DORI: Christina Ayala, DORI Project Manager, trujillc@usc.edu, 323-442-2500.